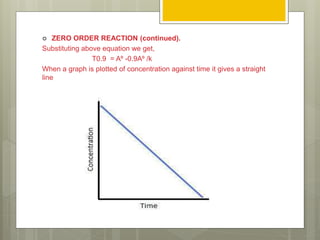
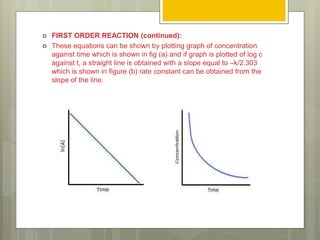
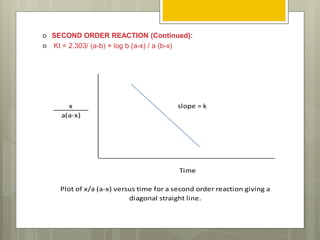
Stability studies are important to ensure drugs maintain their efficacy and safety throughout their shelf life. The rate of a chemical reaction determines a drug's stability and can be zero order, first order, or second order. Various factors influence reaction rates, including temperature, pH, moisture, light exposure, and concentration. Understanding reaction kinetics and identifying the order of a reaction allows researchers to predict a drug's shelf life by substituting experimental data into the appropriate rate equation.