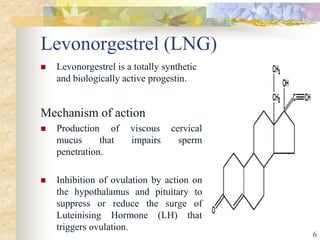
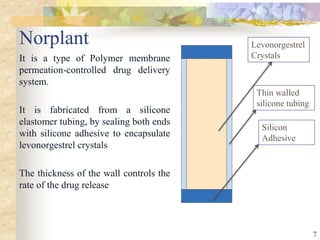
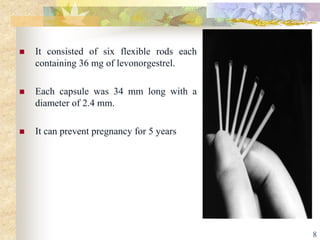
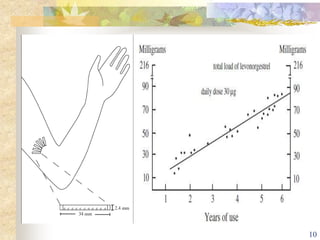
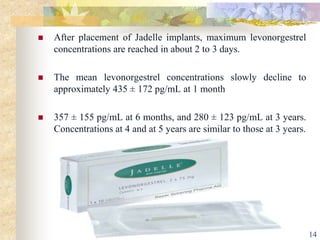
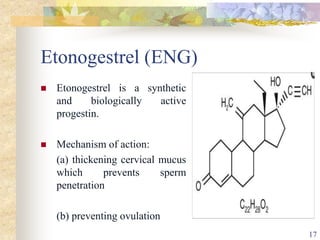
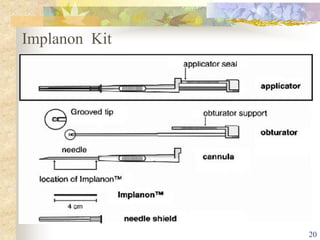
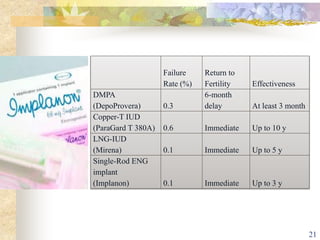
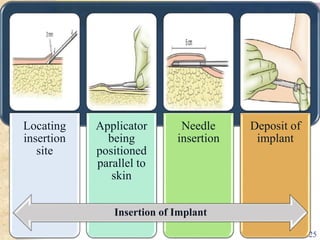
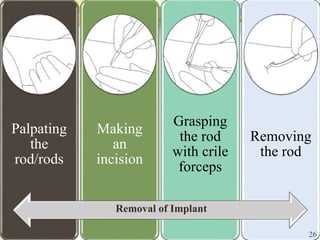
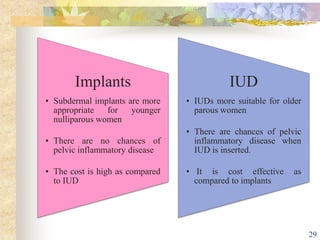
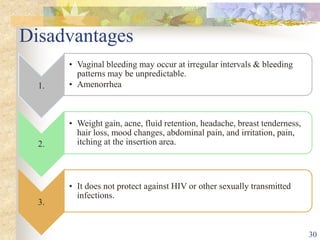
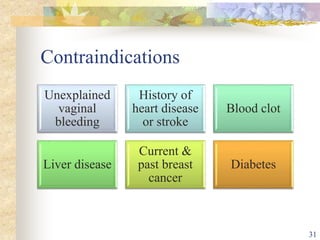
This document summarizes various contraceptive implant methods. It describes the types of contraceptive implants including Norplant, Jadelle, Sino-implant, Implanon and Nexplanon. It explains that the implants work by releasing progestin hormones like levonorgestrel or etonogestrel over a period of years. The document discusses the mechanisms of action, effectiveness rates and side effects of the implant methods. It also covers insertion and removal procedures as well as advantages and disadvantages of contraceptive implants.