The document discusses drug stability and International Conference on Harmonization (ICH) guidelines for stability testing. It provides details on:
1) Chemical kinetics and reaction order (zero, first, second, pseudo-first order) and how they impact degradation rate calculations.
2) Factors that influence drug stability like temperature, solvent, ionic strength, dielectric constant, and acid/base catalysts.
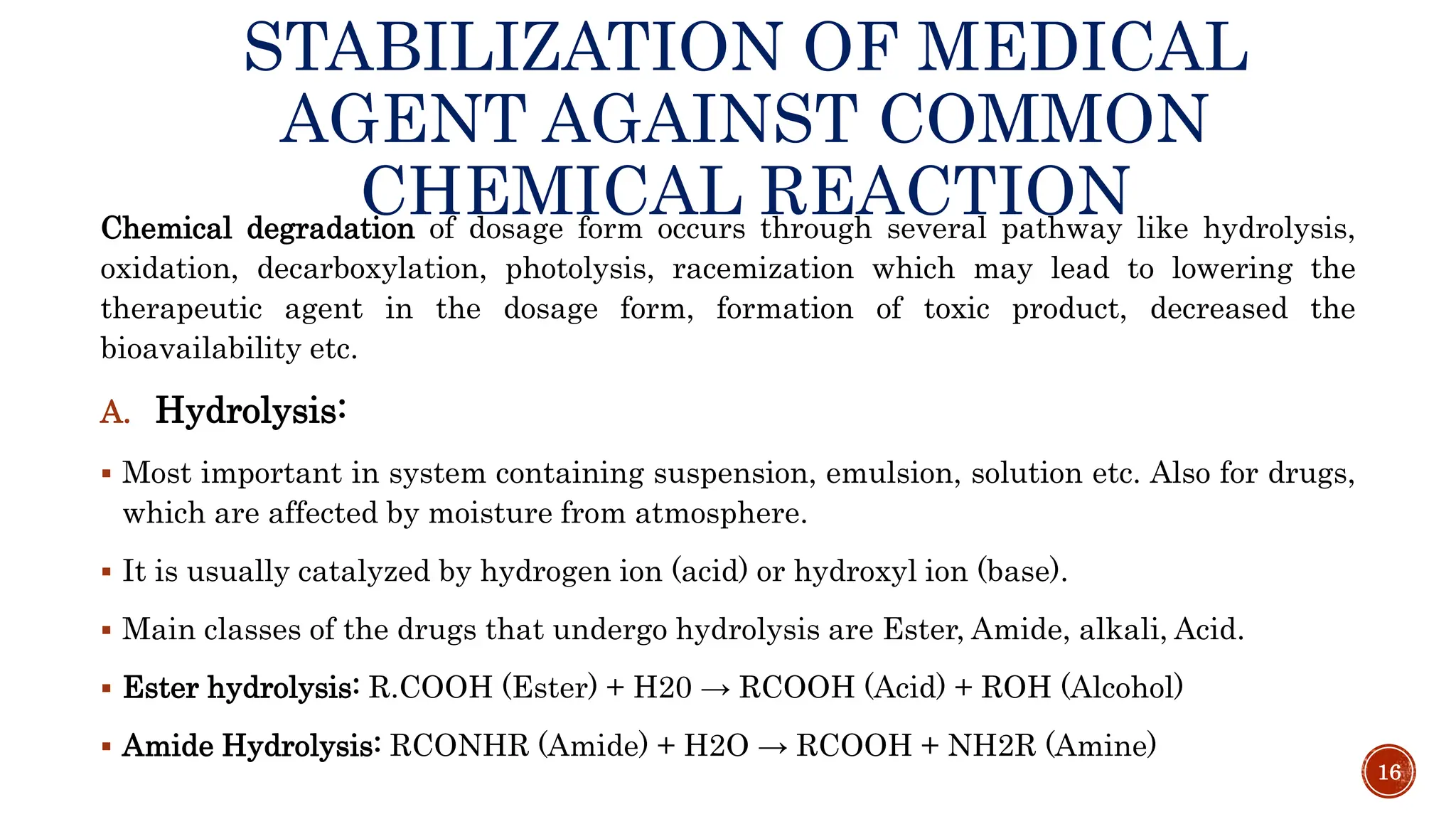
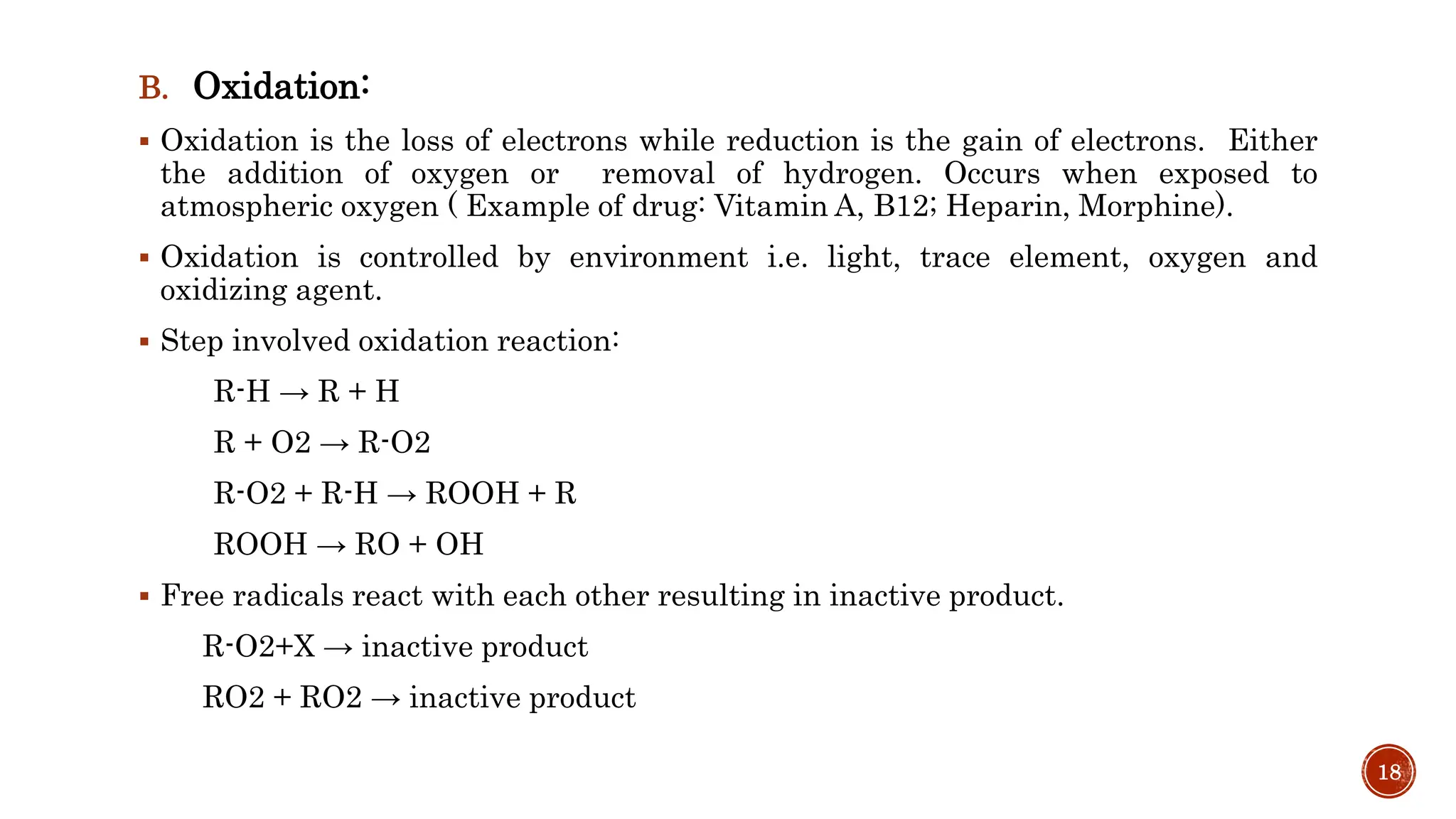
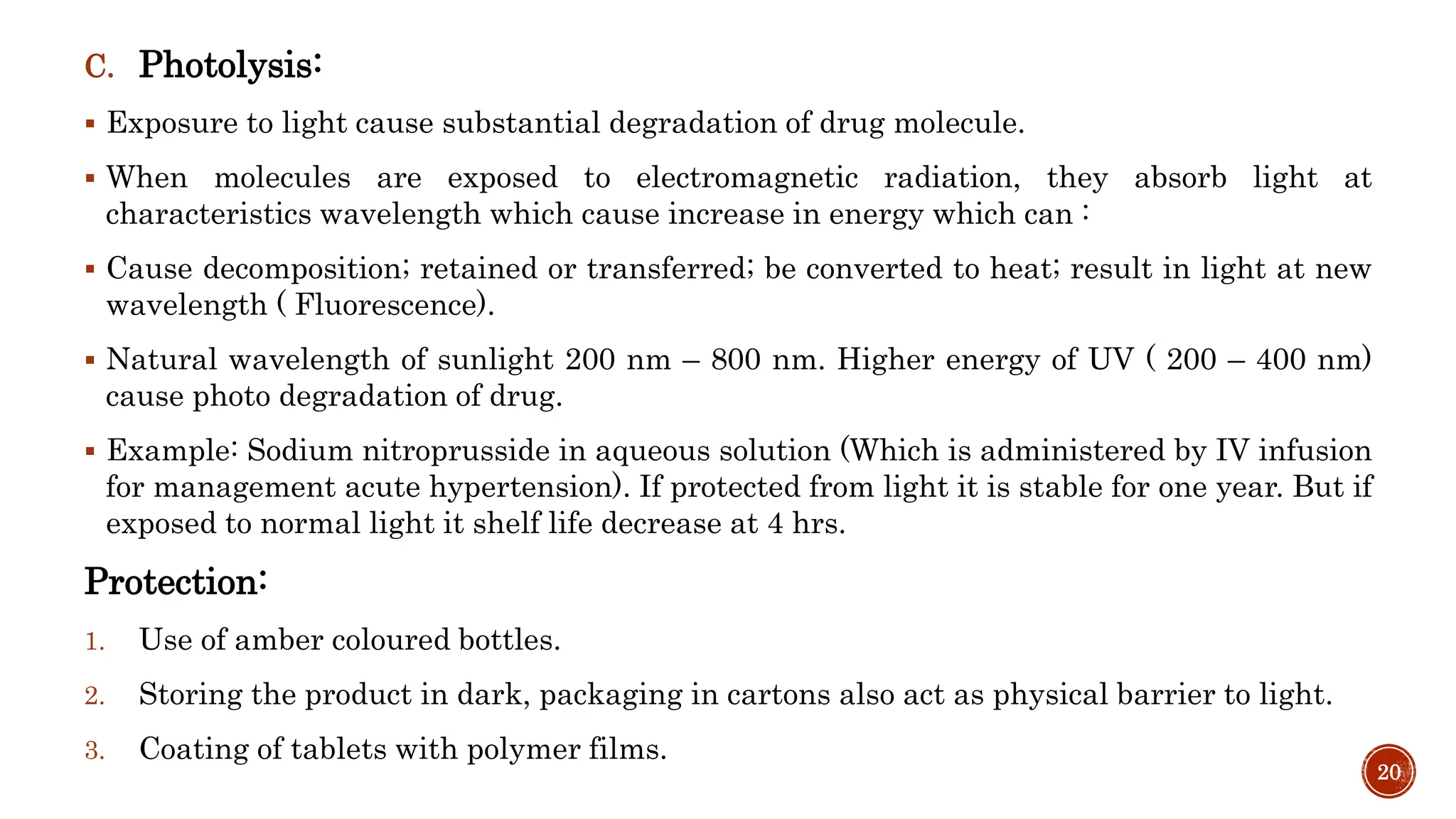
3) Common degradation pathways like hydrolysis, oxidation, photolysis and methods to prevent or minimize degradation through each pathway.
4) ICH guidelines divide stability testing guidelines into quality, safety, efficacy and multidisciplinary categories to ensure drug quality and efficacy globally.

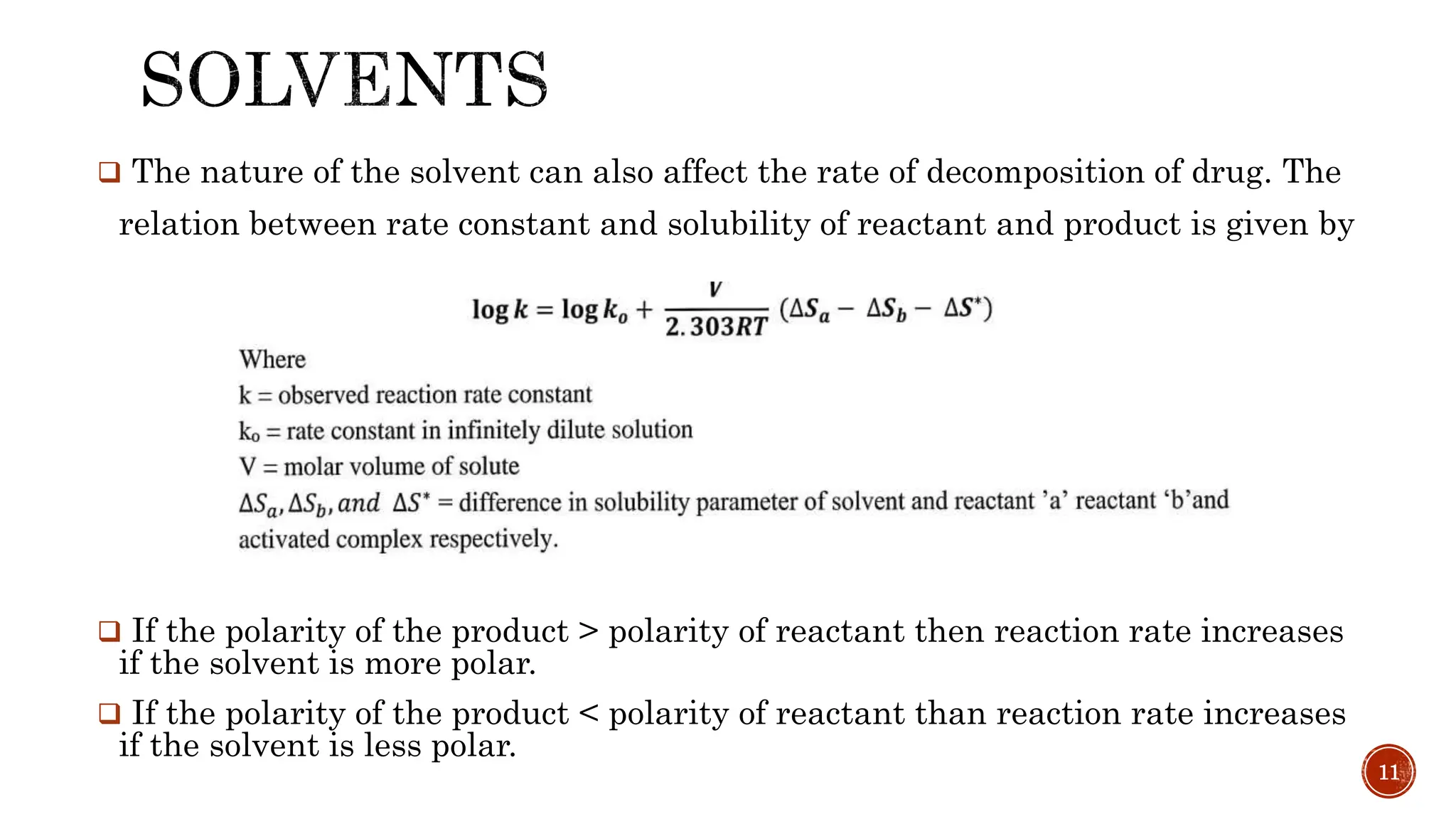
![DRUG STABILITY
Chemical Kinetics:
It deals with the rate, velocity or rate of reaction at which chemical reaction occurs.
Rate of Reaction =Change in Conc. of reactant/ time = dc/dt
Order of reaction:
It is define as the number of concentration terms on which the rate of reaction depend when
determined experimentally.
Zero order of reaction:
When the reaction rate does not depend on the concentration of reacting substance (i.e. rate
depends on zero power of reactant).
[A]0 + [B]0
------------ Product
−
𝑑𝑐
𝑑𝑡
= 𝑘
2
Where,
c is the conc. of reactant
k is Rate constant
initial conc. Co time t0
Final conc. C time t
or, 𝐶𝑜
𝑐
−𝑑𝑐 = 𝑡𝑜
𝑡
𝑑𝑡
or, -c + c0 = kt
or, k =
𝐶0 −𝐶
𝑡
Unit of Zero order reaction: mol. L-1.S-1
Integration both side](https://image.slidesharecdn.com/unit-5physicalpharmacy-iidrugstability-231103052745-4985b4ba/75/Unit-5-Physical-Pharmacy-II-Drug-stability-pptx-2-2048.jpg)
![First order of reaction:
When the rate of reaction depends on concentration of one reactant.
[A]1 + [B]0
------------ Product
-
𝒅𝒄
𝒅𝒕
∝ 𝒄
or, -
𝑑𝑐
𝑑𝑡
= kc
or, - 0
𝑐 𝑑𝑐
𝑑𝑡
= 𝑘 0
𝑡
𝑑𝑡
or, -ln c + ln c0 = kt
or, log c0 – log c = kt/2.303
or, log
c0
c
= kt/2.303
4
Where,
c is the conc. of reactant
k is Rate constant
initial conc. Co time t0
Final conc. C time t
Integration both side
Unit of First order reaction: S-1
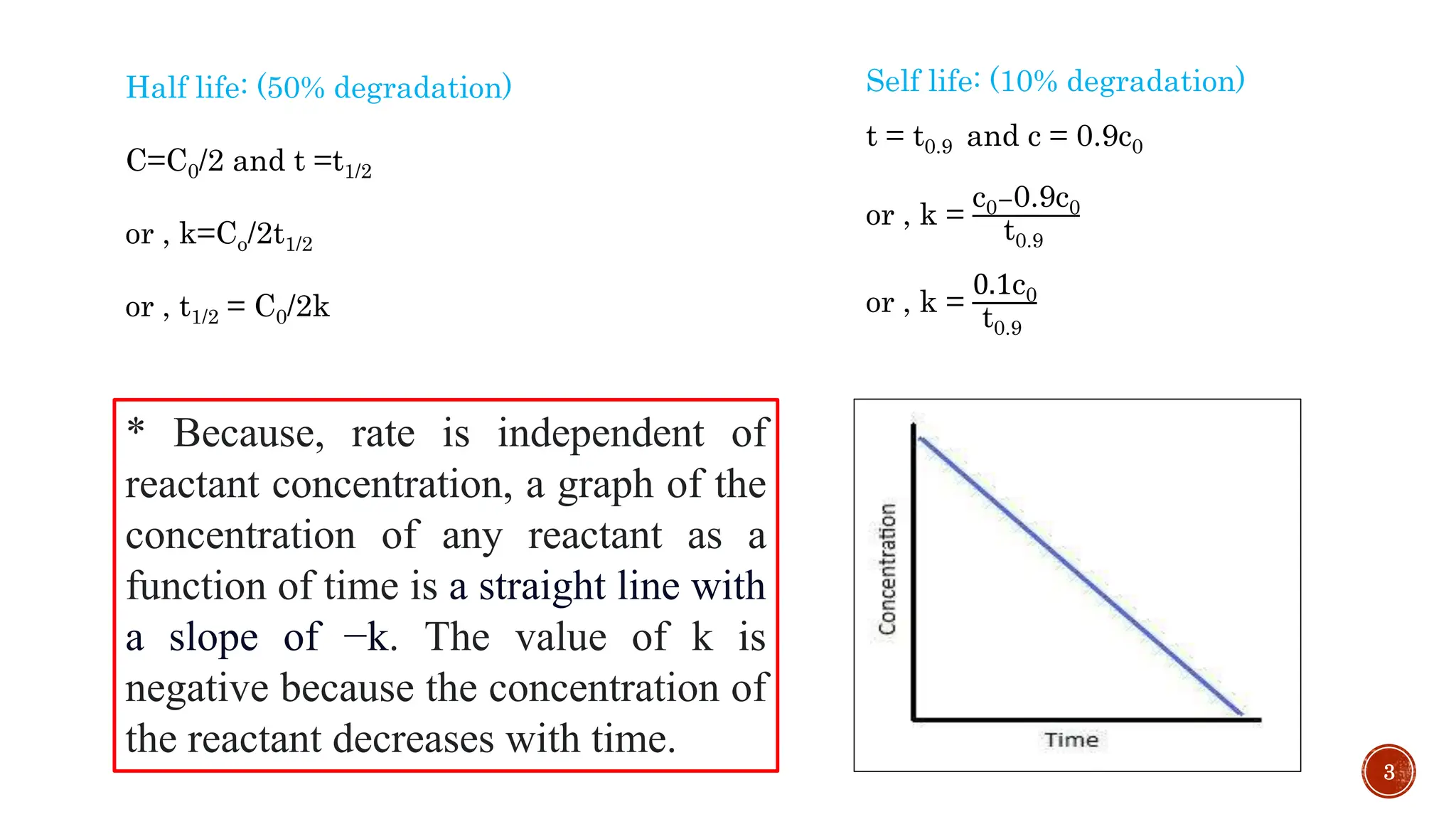
Half life: (50% degradation)
C=C0/2 and t =t1/2
log
c0
C0/2
= k t1/2 /2.303
or, t1/2 = 0.693/k
Self life: (10% degradation)
t = t0.9 and c = 0.9c0
k =
c0−0.9c0
t0.9
k =
0.1c0
t0.9
* The concentration v/s time graph for a first-order reaction is provided
below. For first-order reactions, the equation log c = -kt/2.303+ log c0 is
similar to that of a straight line (y = mx + c) with slope –k/ 2.303. This
line can be graphically plotted as follows](https://image.slidesharecdn.com/unit-5physicalpharmacy-iidrugstability-231103052745-4985b4ba/75/Unit-5-Physical-Pharmacy-II-Drug-stability-pptx-4-2048.jpg)
![Second order of reaction :
The reaction rate of a chemical reaction in which the rate is proportional to the product of
the concentrations (in moles) of two of the reactants (also called bimolecular kinetics), or to
the square of the molar concentration of the reactant.
i. [A]2 + [B]0
------------ Product
ii. [A]1 + [B]1
------------ Product
If (i) a=b
At time = 0
Initial conc. of [A] = a
Initial conc. of [B] = b
At time t,
x = degradation conc.
degradation conc. of [A] = (a-x)
degradation conc. of [B] = (b-x)
5
𝒅𝒙
𝒅𝒕
= k [A] [B]
or,
𝑑𝑥
𝑑𝑡
= k (a-x)(b-x)
or,
𝑑𝑥
𝑑𝑡
= k (a-x)2 (a-x) = (b-x) [a=b]
or,
𝑑𝑥
(a−x)2
= k dt
or, - 0
𝑥 𝑑𝑥
(a−x)2
= 𝑘 0
𝑡
𝑑𝑡
or,
1
(𝑎−𝑥)
-
1
(𝑎−0)
= k(t-0)
or,
𝑥
𝑎−𝑥 𝑎
= kt
or, k =
𝑥
𝑎𝑡(𝑎−𝑥)
Integration both side
Unit of Second order reaction: L. mol-1. S-1](https://image.slidesharecdn.com/unit-5physicalpharmacy-iidrugstability-231103052745-4985b4ba/75/Unit-5-Physical-Pharmacy-II-Drug-stability-pptx-5-2048.jpg)





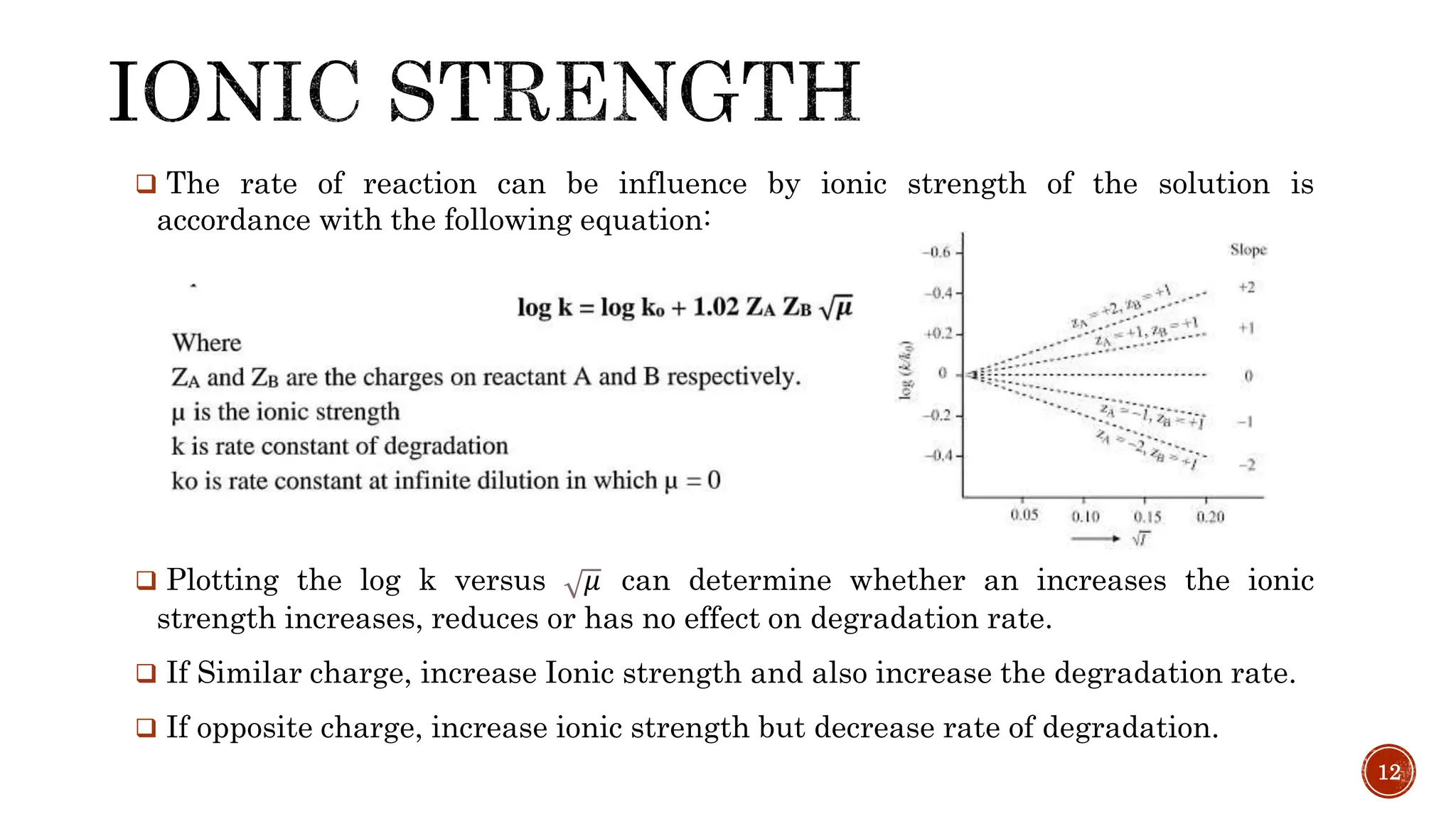


![ Specific acid-base catalyst:
The number of drugs become posed on the addition of acid alcohol base. When the
rate law for an accelerated decomposition reaction contains a term involving the
concentration of hydrogen ion or hydroxyl ion, the reaction is called specific acid-
base catalyst.
The general rate law which express the pH dependency Specific acid-base catalyst
reaction.
𝒅𝒙
𝒅𝒕
= 𝒌𝟎 + 𝒌𝟏 𝑯+ + 𝒌𝟐 𝑶𝑯− [𝑺]
At low pH, 𝑘1 𝐻+ > 𝑘2 𝑂𝐻− and 𝑘0 because the conc. of hydrogen is high, and
specific acid catalysis is observed.
At high pH, 𝑘2 𝑂𝐻− > 𝑘1 𝐻+ and 𝑘0 because of presence of high conc. of hydroxyl
ion, and specific base catalysis observed.
15](https://image.slidesharecdn.com/unit-5physicalpharmacy-iidrugstability-231103052745-4985b4ba/75/Unit-5-Physical-Pharmacy-II-Drug-stability-pptx-15-2048.jpg)