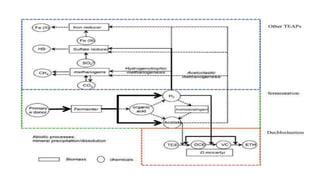
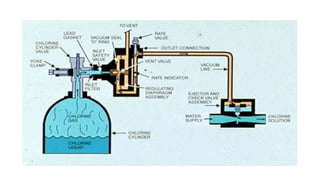
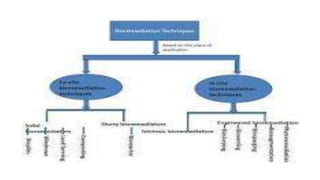
This document discusses various types and methods of dechlorination and chlorination used in water treatment. It provides details on in situ dechlorination of polychlorinated biphenyls in soils and sediments. The types of dechlorination discussed include sulfur dioxide dechlorination, sodium bisulfite dechlorination, and sodium metabisulfite dechlorination. The document also covers types of chlorination processes like plain chlorination, pre-chlorination, post-chlorination, double chlorination, and breakpoint chlorination. The advantages and disadvantages of in situ and ex situ chlorination methods are summarized.