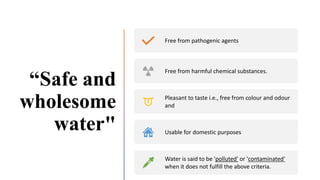
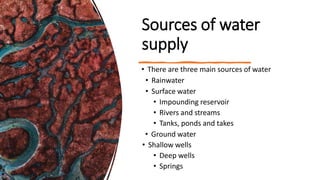
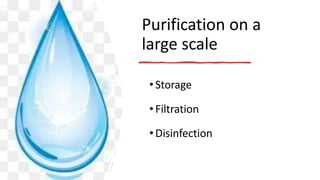
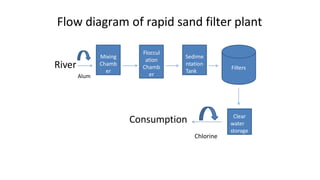
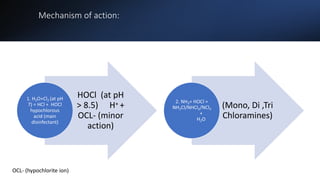
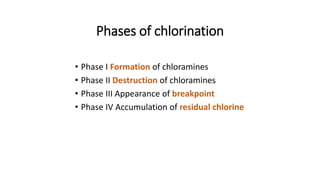
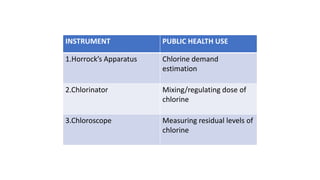
The document outlines the importance of safe drinking water and the processes involved in water disinfection, particularly through chlorination. It details the sources, purification methods, and specific phases of chlorination, including using chemicals like chlorine and its compounds to eliminate harmful microorganisms. Additionally, the document covers practical applications, testing methods for chlorine demand, and guidelines for effective water treatment.