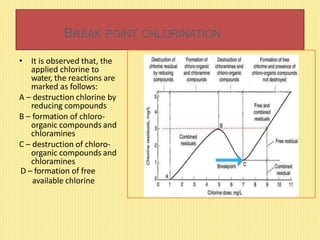
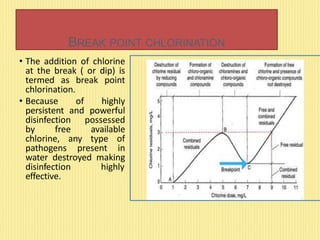
This document discusses disinfection and chlorination of water. It describes different disinfection methods like chlorination, ozonization, and UV rays. Chlorination involves adding small doses of chlorine or chlorine compounds to water to kill bacteria. The document discusses chlorine dosage, factors affecting chlorination, and special chlorination methods like pre-chlorination, double chlorination, and break point chlorination which involves adding chlorine until all organic matter is oxidized leaving residual chlorine.