Embed presentation
Download to read offline



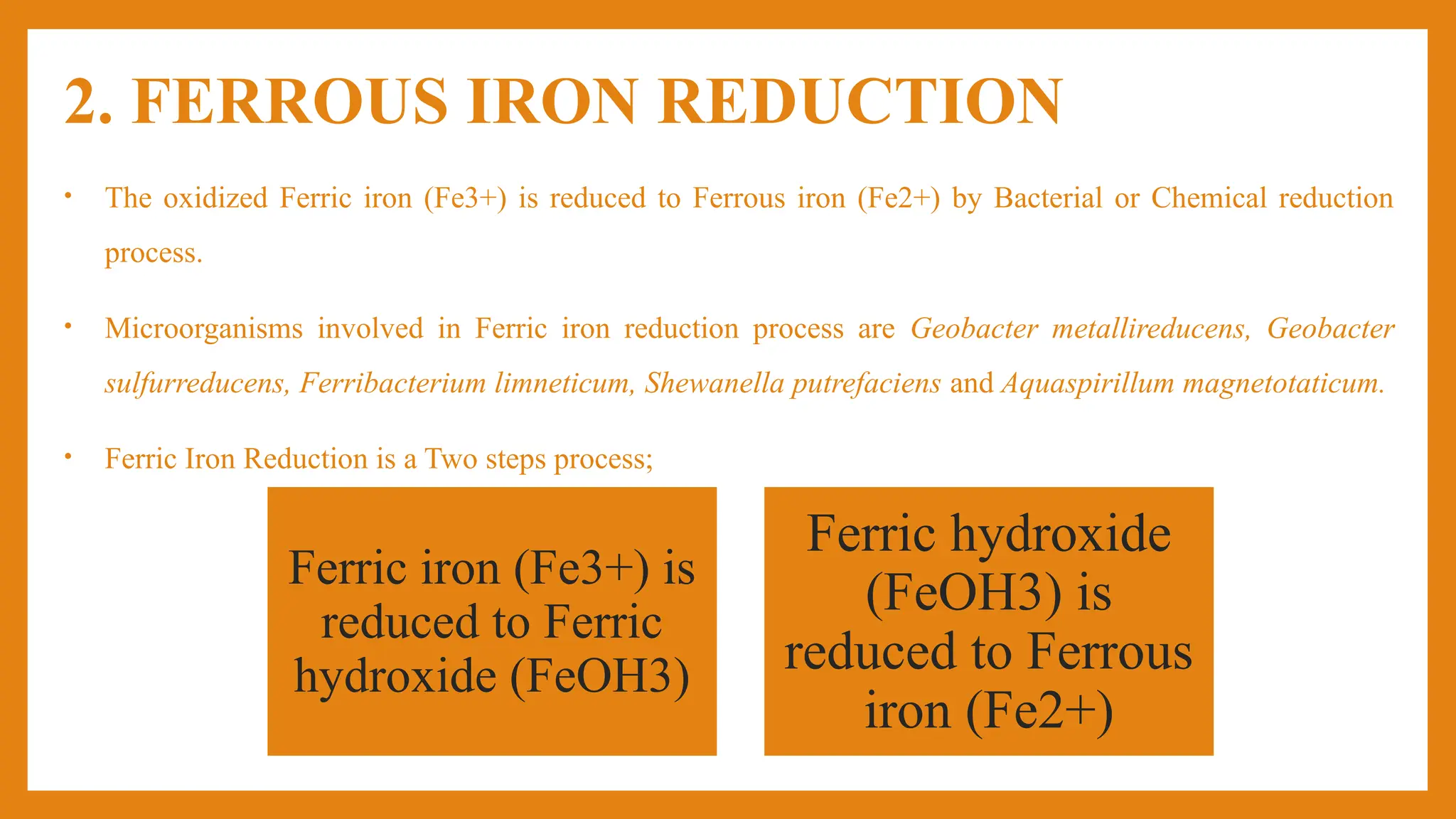


The document discusses the iron cycle, highlighting its importance as one of the most abundant elements on Earth and its various forms in different oxidation states. It describes the processes of ferrous iron oxidation and ferric iron reduction, detailing the microorganisms involved in each process. Overall, it outlines the biogeochemical cycling of iron through various spheres of the Earth.






