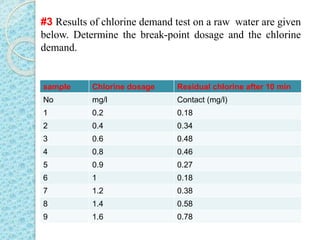
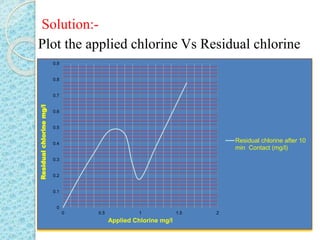
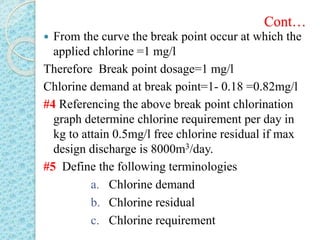
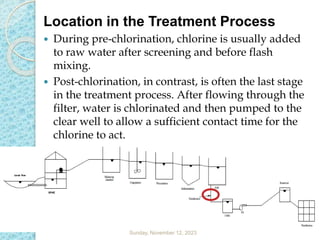
The document discusses water disinfection and chlorination processes. It explains that disinfection uses physical or chemical processes to inactivate pathogens in water and ensure it is safe to drink. The most common disinfection method is chlorination, which uses chlorine compounds like calcium hypochlorite and sodium hypochlorite. Chlorination is effective at killing microorganisms and provides ongoing protection if a chlorine residual is maintained. The document outlines chlorination best practices like dosage levels, contact times, and achieving the chlorine breakpoint for effective disinfection.



![Methods of Disinfection
The disinfection of water can be done by one of the following
methods:
Boiling of water
Ultra–Violate rays
Iodine and bromine
Ozone O3
In most situations chlorine is selected as one of the disinfecting
agent due to:
Effective at killing micro-organisms
Tasteless and odourless
Non-toxic to human being and others
Easy to handle, transport and apply
Easy to detect
Capable of providing protection against later contamination.
Readily available
Cheap
Excess lime
Potassium permanganate
[KMnO4]
Chlorine
Sunday, November 12, 2023
Detail](https://image.slidesharecdn.com/disinfaction-231112182628-f87be4d3/85/Disinfaction-pptx-5-320.jpg)