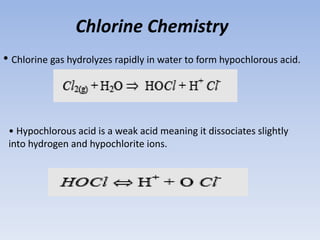
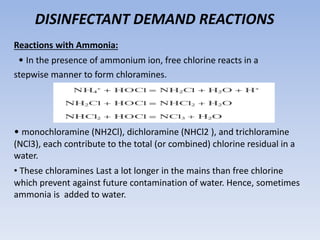
Disinfection is a critical process in water treatment, ensuring the removal of harmful microorganisms to prevent disease. Various methods, such as chlorination, boiling, and the use of ozone or UV rays, are employed to disinfect water, each with its own advantages and limitations. Chlorination is the most common method due to its effectiveness, cost-efficiency, and ability to leave residual disinfectant to protect against future contamination.