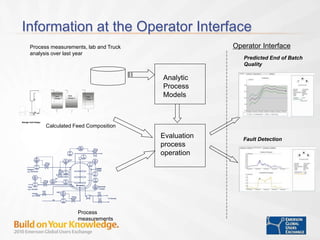
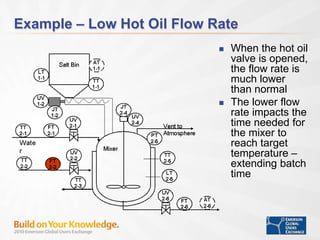
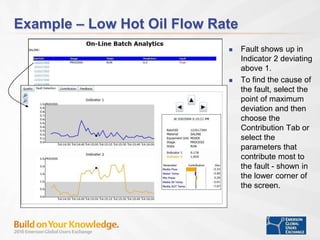
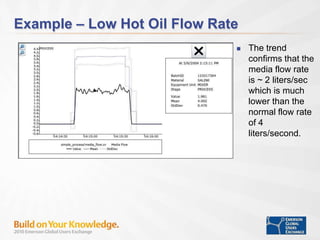
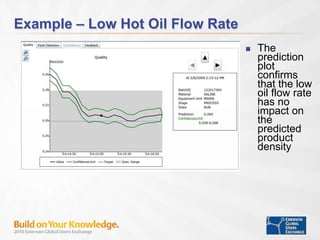
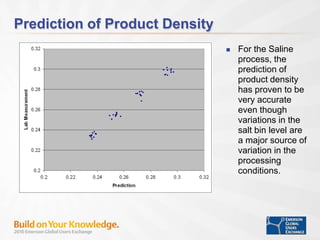
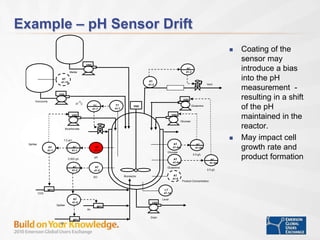
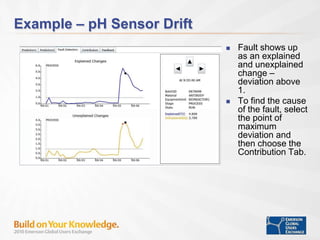
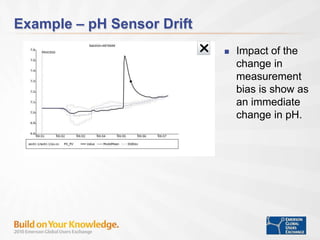
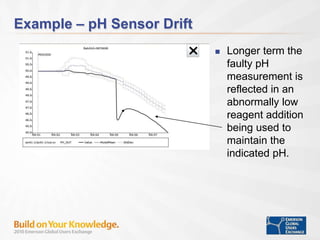
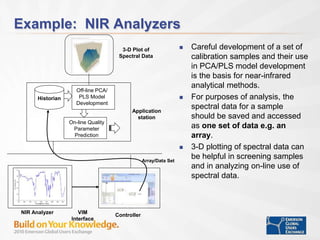
The document discusses the implementation of on-line data analytics and spectral analyzers in operations, focusing on improving product quality and fault detection in pharmaceutical processes. It outlines the use of multivariate analytics as per FDA guidelines, emphasizing the importance of real-time quality predictions and corrective actions based on data trends. Additionally, it highlights various examples of fault detection, such as low hot oil flow rates and pH sensor drift, as well as workshops on these analytics at an upcoming event.