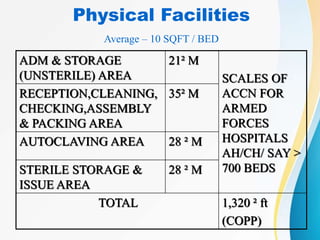
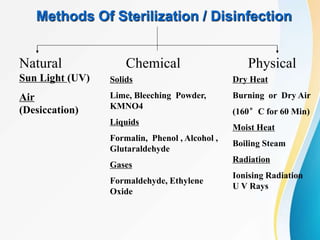
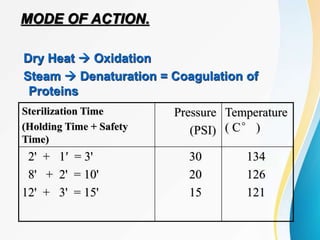
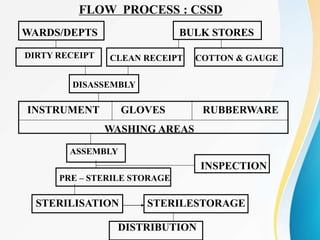
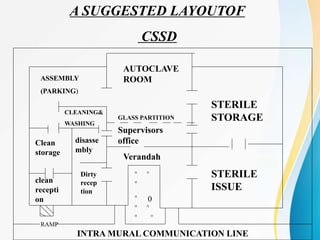
The Central Sterile Supply Department (CSSD) is responsible for cleaning, sterilizing, packaging, storing and distributing medical equipment and supplies. Key functions of the CSSD include decontamination, sterilization using steam or radiation, and distribution of sterile supplies to patient care areas. Strict protocols are followed around packaging, sterilization, and storage to ensure sterility of supplies. The CSSD aims to provide sterile materials efficiently while reducing burden on nursing staff.







![Steps in the Decontamination Process
• Transport
• Attire
• Sorting
• Soaking
• Washing [Detergent, Equipment, Ultrasonic, Inspection]
Types of Packaging
• Textiles
• Nonwovens
• Pouch packaging
• Rigid container systems](https://image.slidesharecdn.com/cssd-230127114805-f6ae71a8/85/CSSD-ppt-8-320.jpg)