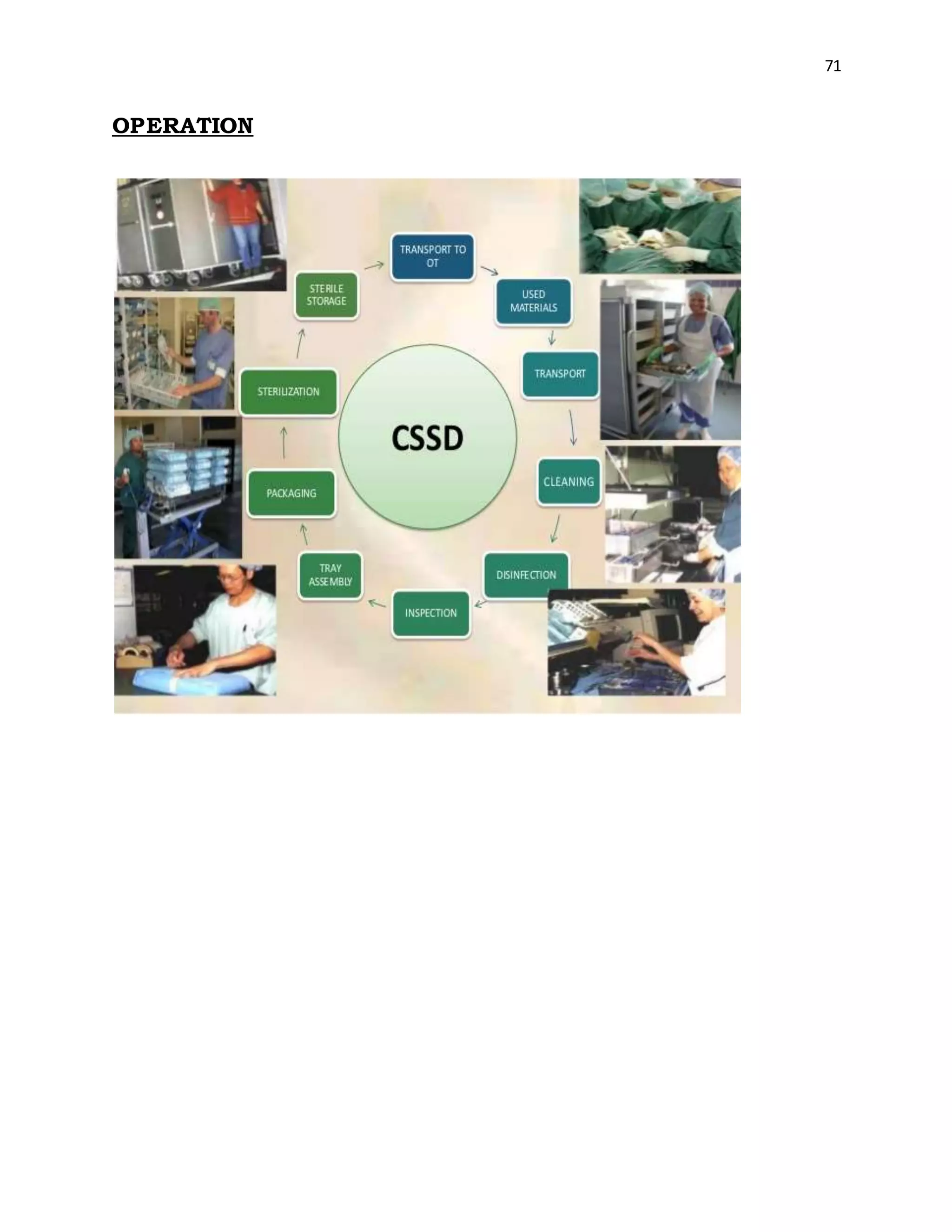
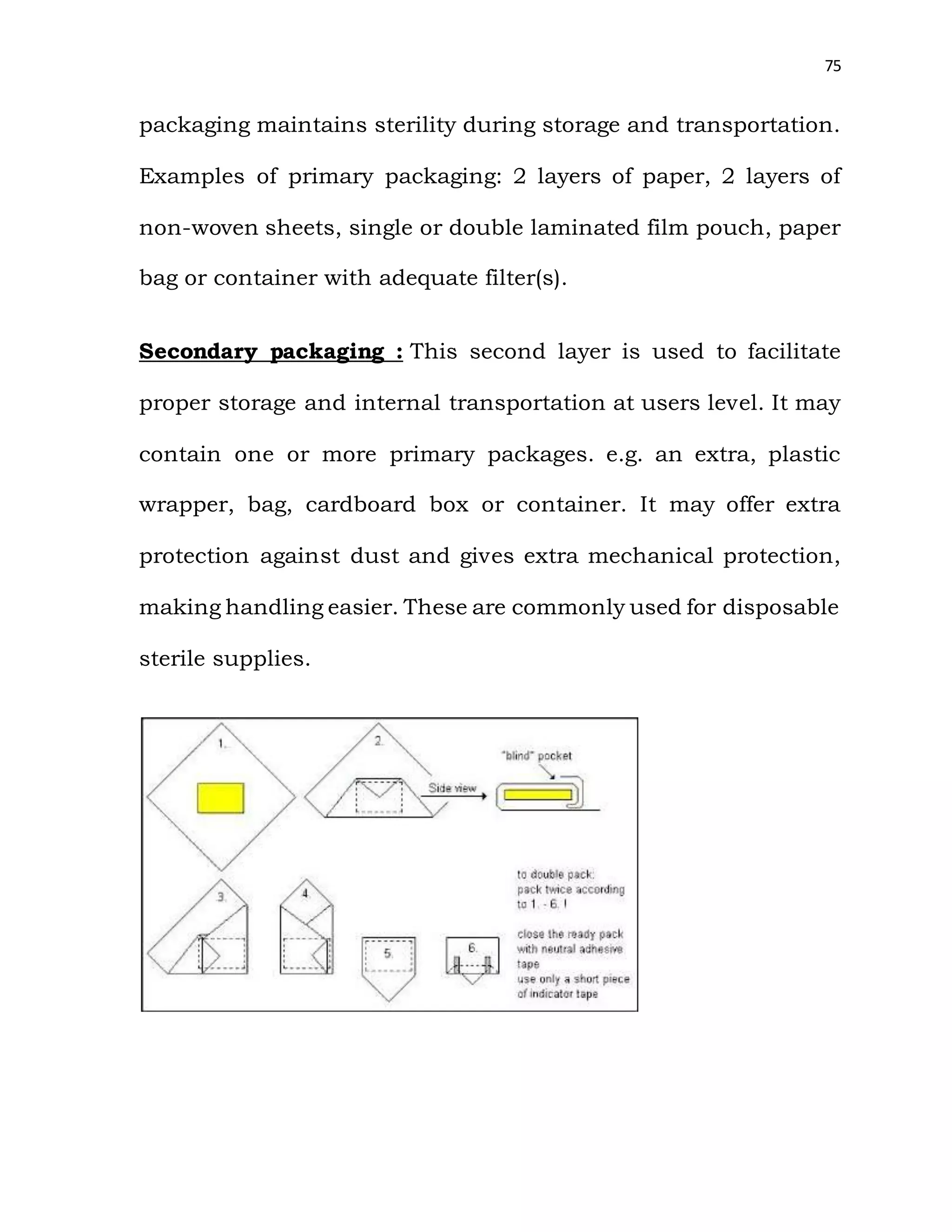
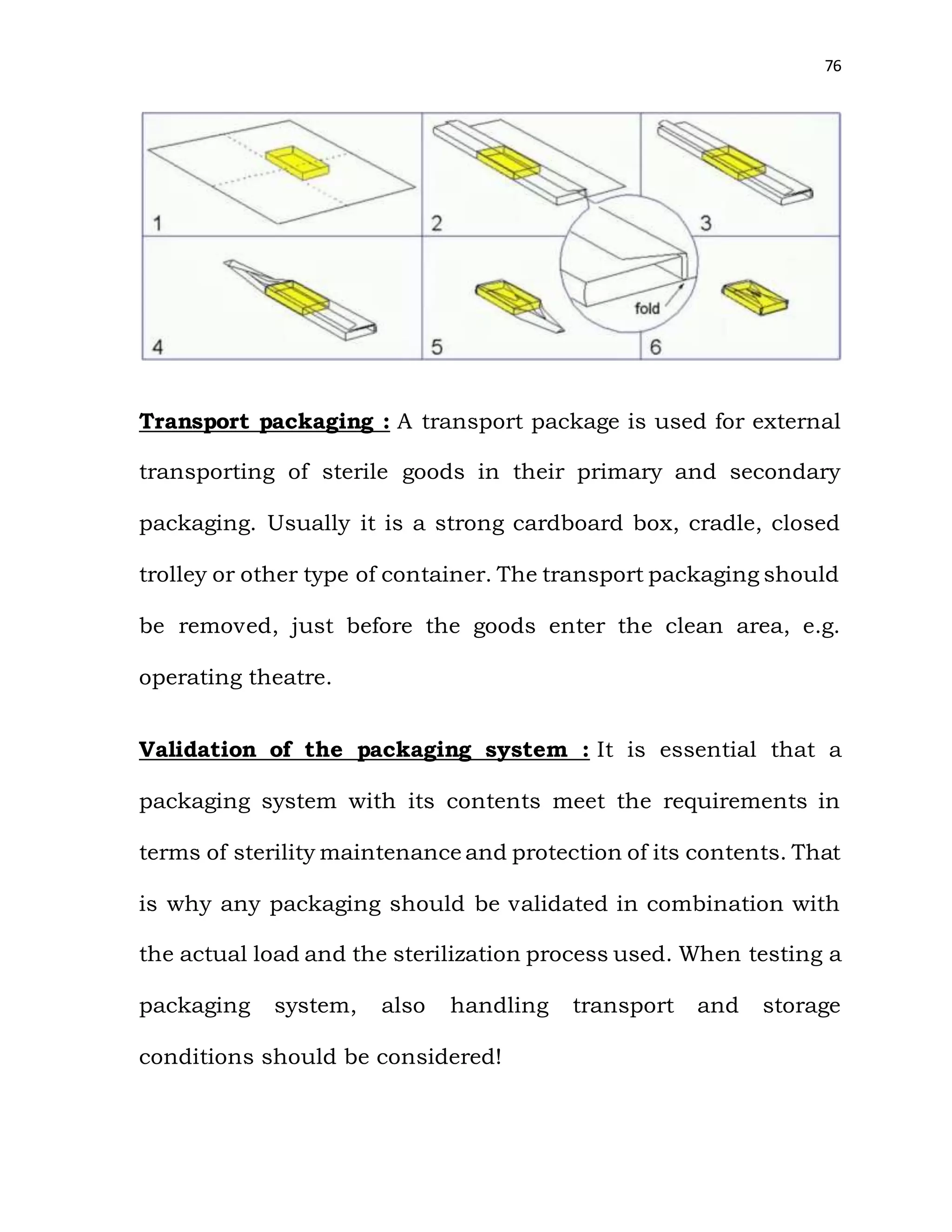
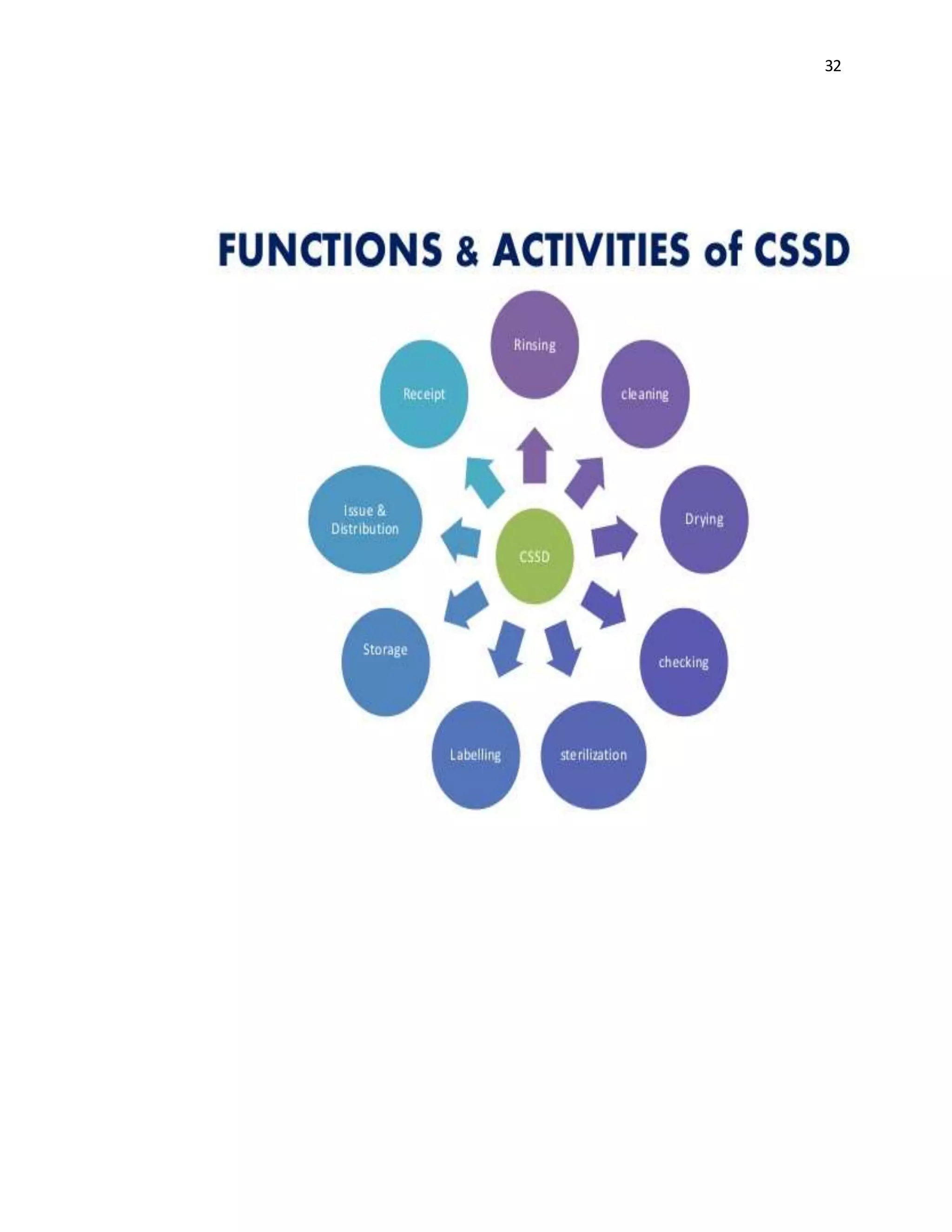
The document is a project report submitted by Megha Milind Sawant for the partial fulfillment of an MBA degree. It discusses conducting a study on the efficiency of the Central Sterile Supply Department (CSSD) in a multispecialty hospital. The CSSD is responsible for sterilizing medical equipment and supplies before use. The objectives of the study are to develop safety checklists for the CSSD and ensure quality sterilization processes to reduce hospital infections and improve patient care. The report provides background information on the CSSD, including its functions, policies, history and objectives.










































![46
The Grocery System: The departments send their requisition to
CSSD from where deliveries are made in accordance with the
demand. This system is sometimes not practical as some
departments demand the entire amount of stock from the
CSSD having nothing left for the others. The efficiency of the
system depends on the wisdom and experience of the person
making the indent.
The Clean for Dirty Exchange System: According to this
system one clean article is issued for each dirty returned to
the CSSD.
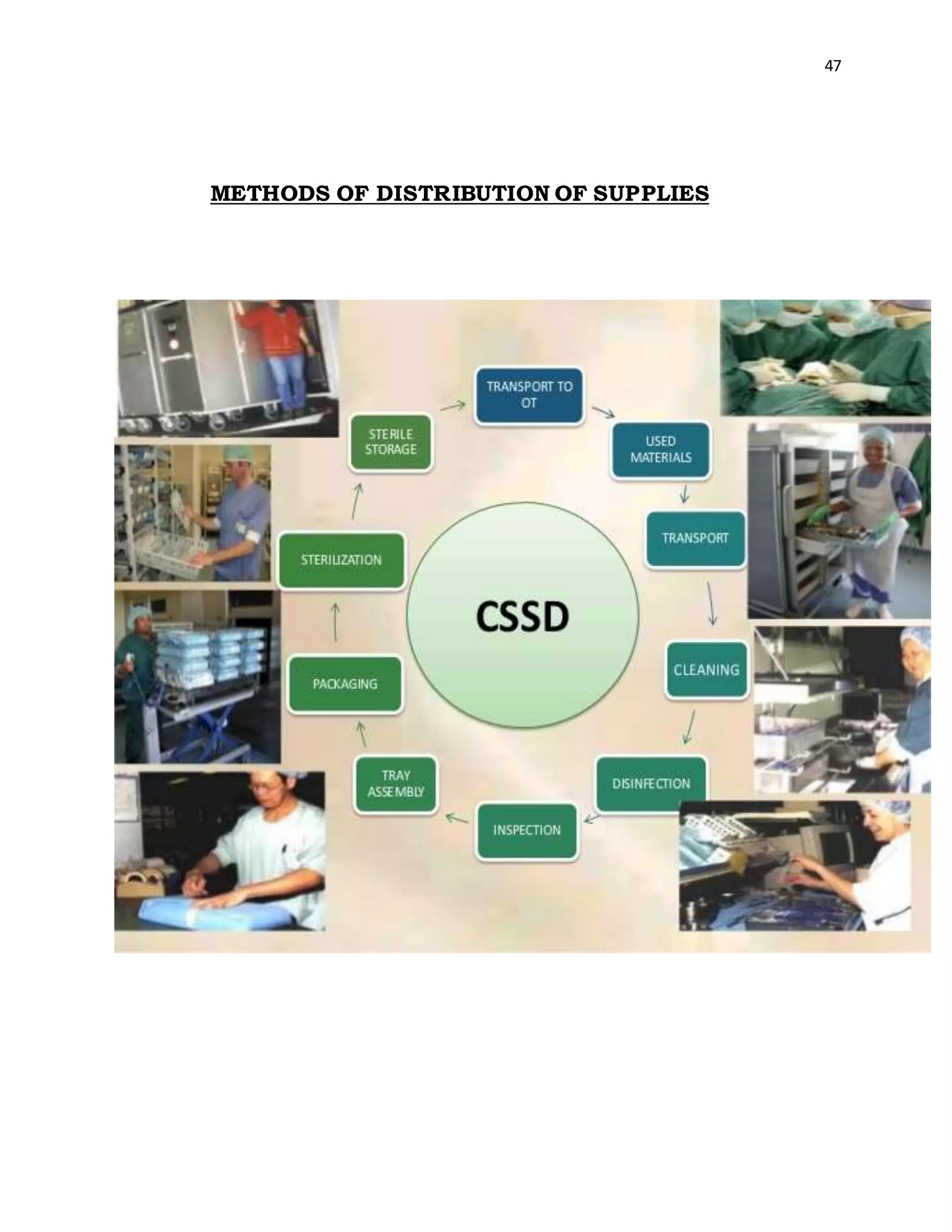
The Sterile Supply Department within a hospital receives
stores, sterilizes and distributes to all departments including
the wards, outpatient department [OPD] and other special
units such as operating theatre [OT]. Major responsibilities of
CSSD include processing and sterilization of syringes, rubber
goods [catheters, tubing], surgical instruments, treatment
trays and sets, dressings etc. it is also responsible for
economic and effective utilization of equipment resources of
the Hospital under controlled supervision](https://image.slidesharecdn.com/astudyonsafetyqualitycareincssd-201108070957/75/A-study-on-safety-quality-care-in-cssd-46-2048.jpg)