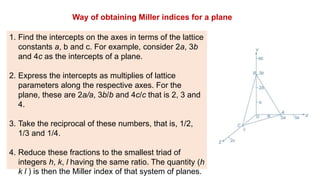
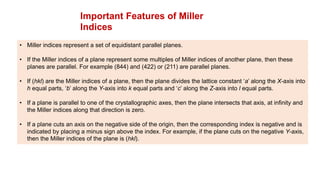
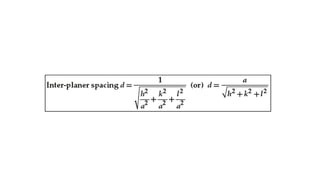
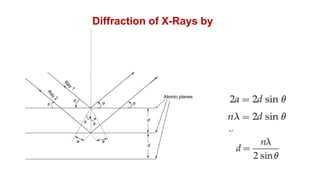
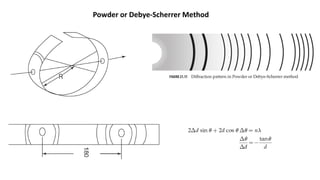
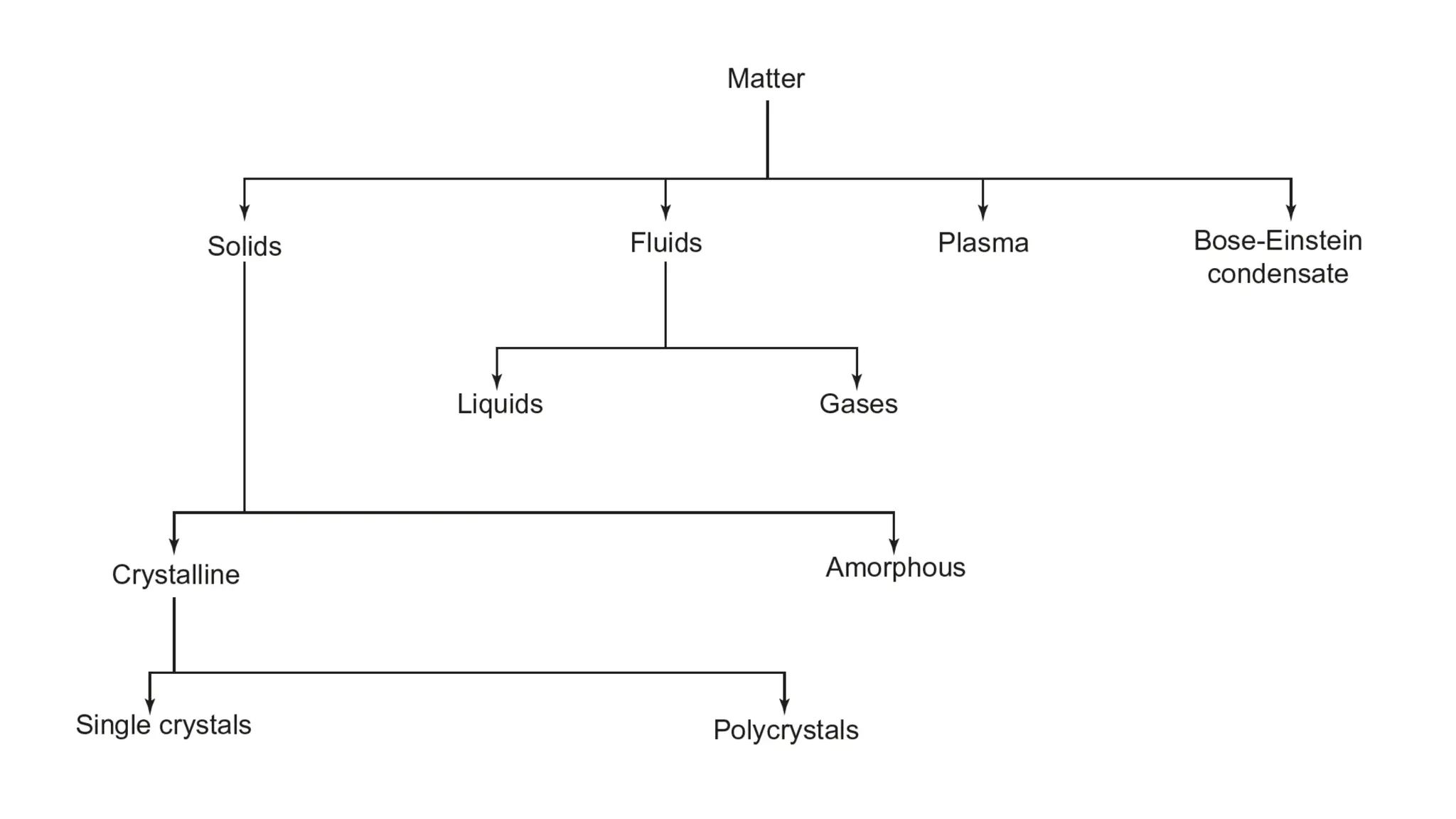
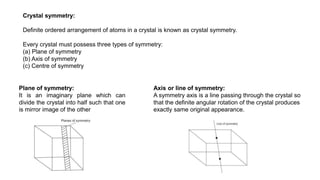
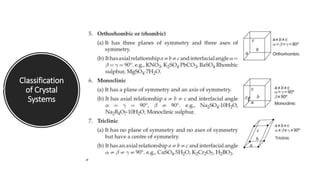
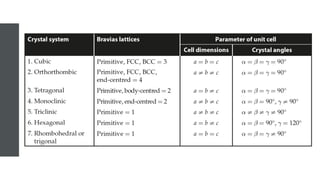
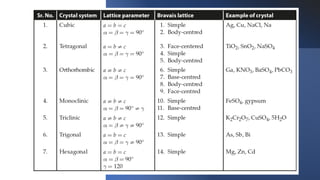
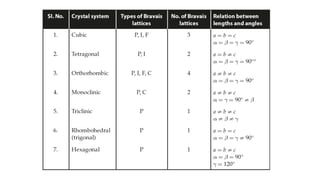
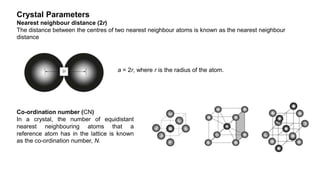
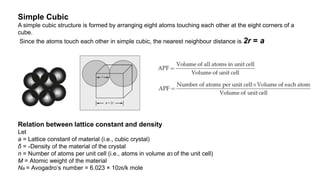
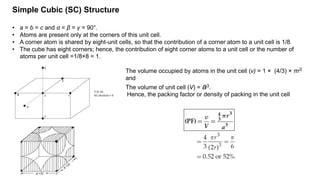
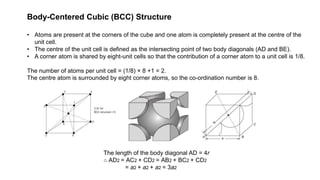
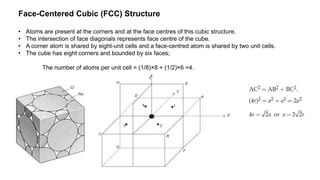
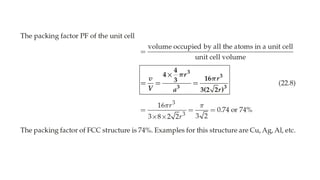
The document provides an overview of crystallography, detailing the geometrical properties, structures, and classifications of crystals and their systems. It explains key concepts such as unit cells, crystal symmetry, coordination number, and packing factors, alongside methodologies for determining Miller indices and interplanar spacing. Additionally, it discusses various crystal structures like simple cubic, body-centered cubic, and face-centered cubic, outlining their atomic arrangements and characteristics.




![Miller Indices
Miller Indices for Direction in Crystal
Crystal planes are defined as some imaginary planes inside a crystal in which large concentration of atoms is
present. Inside the crystal, there exists certain directions along which large concentration of atoms exists. These
directions are called crystal directions.
• Crystal planes and directions can be represented by a set of
three small integers called Miller indices.
• These integers are represented in general as h, k and l.
• If these integers are enclosed in round brackets as (hkl),
then it represents a plane.
• If they are enclosed in square brackets as [hkl], then it
represents crystal direction perpendicular to the above-said
plane.](https://image.slidesharecdn.com/crystallographysp-240101061955-21e925d8/85/Crystallography-SP-pptx-22-320.jpg)