The document discusses coulometry, a quantitative analytical method based on measuring the quantity of electricity that passes through a solution during an electrochemical reaction. It explains the principles of primary and secondary coulometric analysis, highlighting their relevance in measuring chemical quantities, and describes the conditions under which these analyses operate. The document also outlines the necessary equipment, such as coulometers and electrodes, used in these analytical methods.



![-Coulomeiru
I .,,.]
t?D1u(.,..moa» I f I
M , /! .1rn
elf c! r~ () ', ...
Kusha!
-( A,c
the
. . cvncJ.l[ ··r~ .
1 he 11o;u,-:fhus coulometnc methods are those -analyS1s methods which are based on
measurement of.1:he quantity of~l~ctricity,and-the application of the above equation.
~ .
-LJ..aA
A =Atomic weight of the element,
Q =Coulombs of electricity,pa .. Kd -l~11r u h Lt r ~ 1,, -l · • l
n = Valency of the element.
=Weight of the element, cl r -( d ,.,,, -If( (cc f' cl(wl Here,
AQ
W = _n_x_96_5_0_0
) ' ; c.
From this dlscusslon it ;[We"tt-ev.ident-that, if 'o'.'coulombs of electricity have been passed· '.' r• •c J
the solutionlJt~1'weight of the selutien-0Hh~lement1W1'deposite~ will be expressed by
the following equation: l ~ (I) J-
Secondary coulometric anaf.y_sis: (iy-, r1 ,·, ·1<1 r r ~'Lorine-I, , r1 r ,,, ' 1 , r''
The substance
10;.if°czr~~ fz~~~s quantitatively -ffi-st>MicH:t-jVith ~L~i..n~c17~}lrpd 7t(°h., 1 !tr,,.( .f ?
electrolysi . This method is called 'secondary coulometric analysis I ne 'ofThe troduct' rr,{ 11 I
• • (5u} J' c..fr1'1
reacts-Withlhe_s_uhstance...to-be-estim-atecl-anrl-the process is carried11unde~constant
controlled condition ancfa»ri end point indicator is also required.
I[ 00 2.
11 There are two kinds of coulometric analysis:
c ~ . I .!'"ff' ' Ir , c
,. .
Primary coulometricanalysis: "
The substance of interest maybe oxidized or reduced at one of the electrode of the
· J( </ •
electrolytic cell. This analvsis-sirnplv called primary coulometric analysis': In this analysis
the substance reacts at an electrode which is maintained at~constant potential and-/L-
current gradually decreasecsffi€~he substance is removed from the solution.
l / 69. 1.
0-)Here, Nr =Total number of coulombs used to promote the desired reaction,
Nt =Total number of coulombs passed.
100Nr
Current efficiency= --
Nt
n = number of efectro'!)l:l-SM in the reaction.
The fundamental requireme-nt~/:~~ometric analvsis.Is the electrolysis reaction.proceeds
with 100% efficacy.
' I
I Y' ",:,,;
M = molecular weight,
!':; JMtM
3 / 15
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/coulometry-171222061158/85/Coulometry-Manik-4-320.jpg)

![·I I
11r,1,...,uf1J
Xusfia{ -
' '
' I r
1-4-'l-+'"H • I r • •
L-1-li-+-I-+ ' • • •
~ ..............I.
I
<, .: ,., •--rl ( 1~)
l/{.-/ L c /
AqNC3 Sr uFc r:
Coulometrv
After electrolysis the electrolyte is taken out,
t
j
---~f)
-JL »>:
-~~____________@.o] - 12 Coulometer:
2 g, °61J'-"1iilver Coulometer: ~
It consistsof a platinum or silver vessel which
C, r./ · 1'1
acts as a cathode and OOftuttrttflg-awJ.utiGn--e-f-
g.t<cftJrfl am:4'9-lii (]Yi} .t[(
pure AgN03, aS11electrolyte. At first/:A:gN03 is
._,,Co LU-liiSY'
purified by repeated crystallization from
acidified solution. joUowed ~ fu!)l'cm.
The' ~hl-00.-JigNO(;;[;~~~contain 10-
20gnf$altin 100ml solution.
/) '.)
A rod of pure 'silvef-"'enclosed in a porous
lMl.
supporgacts as-anode.
'*- Oxygen-Hydrogen Coulometer,
.,._ SilverCoulometer,
~ Iodine Coulometer.
There are various types of coulometer:
e.
an b. One of the products n!acts with the-
at sl:lbstanee~5 be estimated and the
precess is carried un~e~ constant
controlled condition aad aR eRd poiRt
im;liGater is also req1.1ired C»JJrW
d. It is a slow process.It is a rapid process.
a. The substance of interest reacts
quantitatively in solution with a single
protfuct of electrolysis. This method is
-~~~ledsecondary coulometric analysis. _/
A<'f'V'- f>t.t1..0S4-u...~'c_ Co<llt>w-..P..~
~wnf CWlll ,j C~vtltJ md'nj .
b. IA--t-his-aAa-1-ys-is-tl=ie--s-tJ bstance reao
eleet1 ode which is maintained
constant potential. ~-te(.,t-r-i•k
c. ln-thi-s-analysis-f)fOees-s-ettffer-rt gradually c. Ifl-this-analysis-process.current-ls-ea=led
decreased since the substance is j '.Ul1d"ei;;constant controlled condition.
removed from the solution.
/. I
( t' '.'
I
f"'
a. The substanceof interest maybe oxidized
or reduced at one of the electrode of the
electrolytic cell. This analysis simply
called primary coulometric analysis.
Primary coulometric analysis
, I 1
c-i'.
j 1)'J
1)-'1 -~
2 g '07. 12 }IDifference between Primary coulometric analysis and Secondary coulornetric
analysis:
Secondary coulometric analysis l
5 / 15
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/coulometry-171222061158/85/Coulometry-Manik-6-320.jpg)
![(
2. Contra (Stential coulometric a11J1/ysiJ:
. --- - I - . J• I
The substance· being-determined reacts ~i,th 100% efficacy at-working elect~ode-~T-he ,.., J
potential ~wl:i1c~s- controlleaT"'The~;r;.pl~tl~·~~f the reaction can be-~tJi~ed by the
current.'decr~asmf$fctctitally to zero.f,The~~u~t~fA~ ~eactan!)can be
c0-luilafed ~- - -- --.......1
.._-:e:;omp.1¬ tte,c1either from( !_he reading of a coulometerJ or by means of a current tlrne
~ating devise, When the current is theoretically zero then the reaction tends to
-completed.- ~Yr
1-
1,.,
~ / , , , -e er- 1 J " r i. 1 • ~ 'fY'1' 1 ~ / oj
CIJ_ 091o({eneral,pro erties of tn(l)-_,eoulometriclan~sis:
8'th, oj thR . ( C1/l1:j~
1 /' Coulometric techniqueifveJaccurate and precise result tharrother classical method.
@'!Coulo-rnl:fr-..tob. :Oi&;>methocbbecomeJ more accurate,becauset~ectrical cu_rrent can be controlled
and measured with greaiprecessionthan{):oll:Htle-ef-sel~tioo~'aLurn£f r>i'c ~d
6l}hfoulometric techniques are suitable for both (outine and remote analysis. .
Couiometrq _,, 1 ; Xusfia{ -
One of the titrant is produced at electrode which reactswith the ion to be estimated ·
a. P.rll!lQry_C_Q!2stant current GCJulemet:FiG-analy.sf.s-;.
1
The element to be esttmated.undergoes direct reaction to the electrode with 100%
current efficiency.
~ Second a constant current eg.ufometr-tc-anu./- -sis:
I
-
In this techniq ~he s3lution o substance to badetermtned.Is electrolyzed. T~e
- ,JQ,I <2<-.fl?d I r. , • (
completeness of the reaction can be -0.e.ter-m.iRed.:illy the visual indicator and.circult is
then.opened. The amount of electricity is derived from th_~pr<l.d..lJ~Lafa111.pere .and time.
- ;This method is also divided into two parts:
"''- ,
,,,g. Constant current coulometric analysis,
~ WRmwpotential coulometric analysis.
0.011~1ed (ccms-tam.l]'
..l: Constant currentsou/Q_metFiG analysis:
There are two distinct types of coulometric analysis:
-f.o9.J2 5aJ
1 faraday of electricity {96500 coulombs)= 107.88gm of Ag+ ion deposited;
2s·o1·1?.
w·e13hD..d gr'vef) ,1,.I
the vessel }s washed, dried and "'Weigl'lt-ed. The increase/ in weight Jnd~her si!ve-i:-/Yj -i-
deposited/~ow, remaining the value of 96500 coulombs which is also called 1 Faraday of /
electricity ~eposited 107.88gm of Ag;).
The quantity of electricity that is passedthrough the solution can be calculated from the
following relationship:
6 / 15
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/coulometry-171222061158/85/Coulometry-Manik-7-320.jpg)
![Xusfia{ -Coufometry
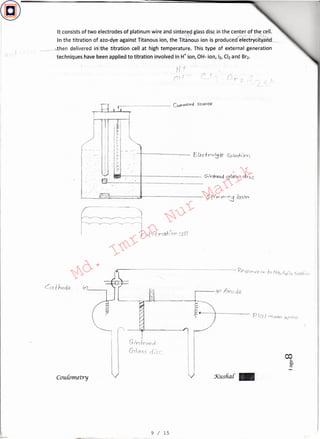
0g 10 /I Intern qigeneration of the titrants:
' » f ·. ',
It is possible to estimate thiosulfate with iodine generated internally with Kl at the anode.I .
This titration cell consists of a generator electrode at which the reagent is formed and
second electrode is used to complete the electrical circuit. An excessof Kl is added to the
thiosulfate solution taken in an electrolytic cell ancfronstant current is allowed to pass
thorough the cell. Iodine liberated at the anode reacts with thiosulfate. The titration can be
One titrant react with analyte-itPer-dinarytltTati'Crn-withthe differenee Jnstead of adding the
titrant from a burette, it is quantitatively generated at an electrode. The titrant substance is
generated either internally or externally.
)·
I
. In coulometric titration small quantities of reagent is required.
I I
- r
·canstant'current coulometry or coulometric titration:
1-, . Standard solutions are not required for the coulometric analysis. The titrant is
~----· ~neratec!_.either in~ernall~~xternall~his.fan give accurate and precise result
_ tha~ ot er classical method. , R. ~ +eclirvauu:
l>-lrirtl-rm Qa.f>ILlJ0<., I
b ·.J:i.tf-ant can be carried out-autcrnatlcallv . .L·
1,·,p. je>~" oonrerr_-lr>-t1.J1on of />Uoo ct.me.Pl)
c. Very small quantitieSICan be analyz~;rby coulometrv.
1,
z .· This method becomes ~ acc~i:te because electrical current can be controlled
and measured with great precession thanvolumeofsolution. - -
5.<!J.r· Coulometric techniques are suitable for both routine and remote analysis.
G. :q' Many titration can be performed coulometrically which cannot be performed by the
classicalmethod. e.g.
~ Titration in melted salt medium,
~ Titration of highly hazardous material,
1*. Titration which utilize unstable titrants such as Br, Cl, Ag, Sn, Cr etc. to workout
analysis.
xJ
k
& , .s.r! {,'I 1 =r":': rrf •d .t; I I. err.cl rrn•iu ,
(1.;,/nt1/u·1•.·; f¥>cz_r(l_j c-l}: -fi'fr>qi1cml) <a-n c,), ~.. 1 '••rrm c ·,... .Le;• J
@.~1anytitration can ~~,performed cou!ometricaliy whic~annot be performed by the
classicalmethod. e.g.
. • • • y( cJtr.·..,.-., • •
~ Titration in melted salt fftea: ·~~~(h-,' .i, Q •rl!!!H,i7"~ ( J •., r
~ Titration of highly hazardous material . ,....
'*- Titration;which utilize%nstable1titrants suc.h a~ Bi;_ Cl12,..A~1~Sn~i-Cr~ .1 e-werkout
ana~y.SiS.· 0C'r> .µJ •.<tf..T 1 c r<t - r,
;Q 112cl · t-0r'deU/ I i- " '.: l •
~ Centro] current technique ha~"been-used than controU'ffotential techniqu5 because
~-h¬ -cor:itr:olcur:r.ent-techr:iiqu~is faster.,aAEl--furequires simplej, ift-f.emiatio+:i and #-is
~Qf f ~1'.,..r;,/n•tm(vr4~1
less expensive. lPJ_QI C ( ..../0 tier/ C (u111lYn./ · ./« n ,':l.._,.,, Ca, h{:
,-rl" -n c<? e:« Jo n-n.m1j , , I - '1.:). c 1 ~'
. . ) lh'C6ulometric titration mall qua~tities of reagentis-r-eft·l!"l·i~ <?y lL 1 c» c 1 tno t: I a -ryJ pncr ,·
- ~ nurt,.Uft( 1.00.12
(}}Advantages of coulometry:
7 / 15
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/coulometry-171222061158/85/Coulometry-Manik-8-320.jpg)

![,...,
~
8
. Post"'~ orvy.,g_~
It requires a potentiostat and chemical coulometer placed in serieswith working electrode.
The chemical coulometer such as Oxygen-Hydrogen coulometer can be used in the circuit to
measure the quantity of electricity. A schematic diagram of the essential component of the
potentiostat is shown in the figure. Passage of current through the chemical coulometer
liberates the H2 at the cathode and 02 at the anode. Both gases are collected and their total
Couiometrv Xusfia{ -
E..9t.h.......:C. 12-l!.~ f-·======t.____<Mlv::::r:~,
i----t-1'-- ~~] - n '---~-~~
_ . C~+i,,.od.e..
e
S'A~•.oul~.:~·;~
ovv-. cL C2.. C2.C?. .- ,f .R.(l_, f
........... - ....._ .. ,Y.:.•:.,.·,~-·-- ...................... ~._ .... '·.:- •:.~
i-----l ?I ...-----..
Controlled potential coulometric analysis:
a. Unavailability of all possible titrants.
Disadvantages of constant current coulometry:
Advantages ofconstant current coulometry:
( l (;
a. The main advaateges-of-the-censtaat.currentcoulometr-y ever-cenventlonel-titratlotr lie
ln.arees-wberelow concentration or unstable titrants are-tnvolved. - ) , I ' '
b. ·The complete titration can be automated with the ease.
c. Standard solutions are not required.
in classicalvolumetric analysis are eliminated in coulometric analysis.
c. The reagents like Cb, Br2, 12 or Ti are inconvenient as volumetric reagents due to their
instability but in coulometric analysis they undergo reaction immediately after
generation.
J ,.,
r:;ibAdvantages of External generation ofthe titrant:
r
a. Sometimes, the internal generation of titrant interferes with the titration. In that case-it , , r
'
I· N is necessaryto generate the.titrant externally.
/b. Problems associated with preparation, standardization and storage of standard solution
10 / 15
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/coulometry-171222061158/85/Coulometry-Manik-11-320.jpg)




