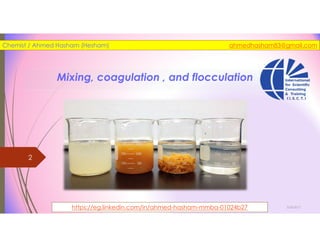
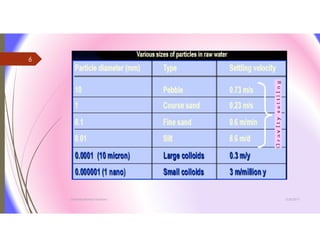
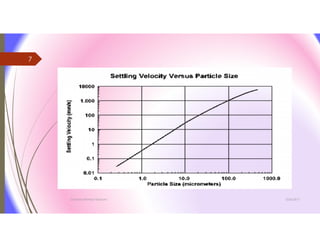
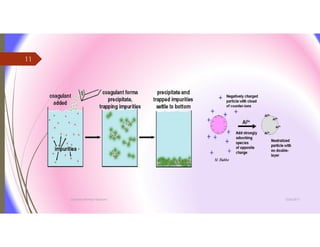
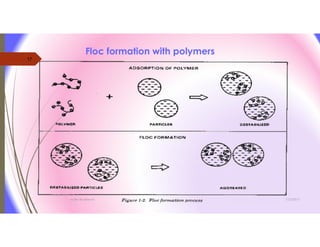
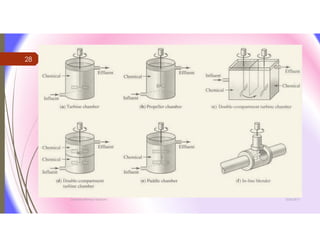
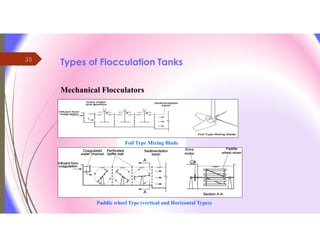
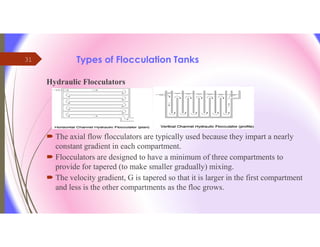
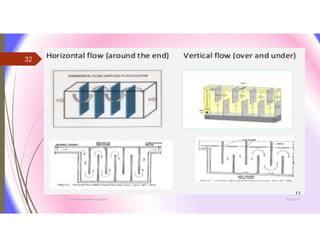
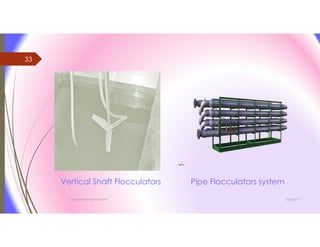
The document discusses the processes of mixing, coagulation, and flocculation in water treatment. It defines coagulation as destabilizing particles in water so they can attach to other particles and be removed. Flocculation is the formation of larger particles called flocs. Key factors that affect these processes are mixing conditions, pH, alkalinity, temperature, and turbidity. Common coagulant chemicals like alum and ferric salts are added for coagulation. Flocculation aids like polymers can help form stronger flocs. Proper process control includes monitoring these factors. Rapid mixing distributes coagulants uniformly while flocculation encourages floc formation.