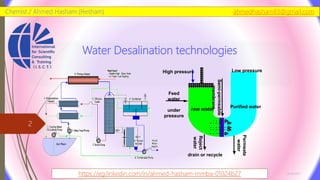
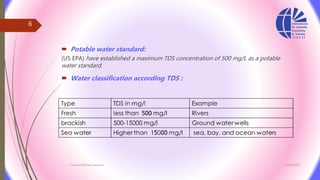
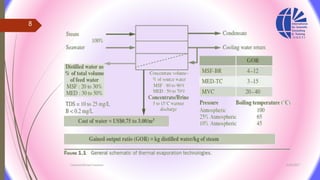
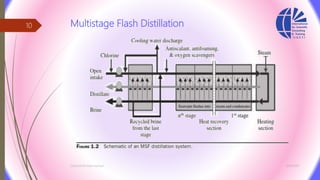
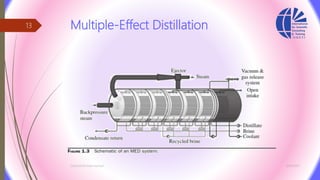
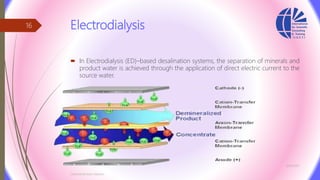
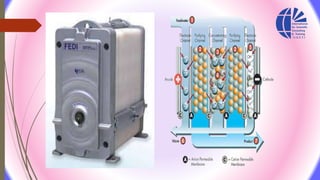
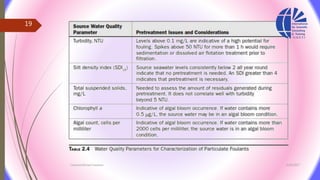
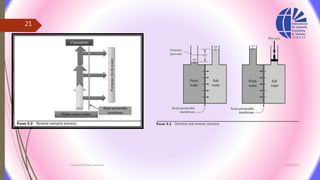
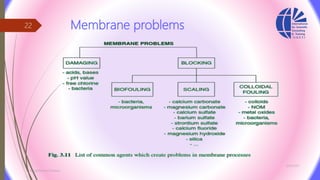
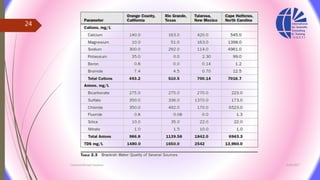
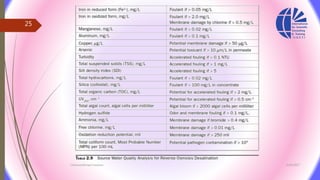
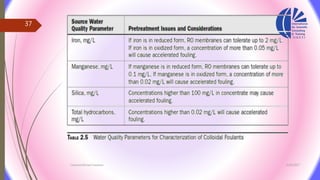
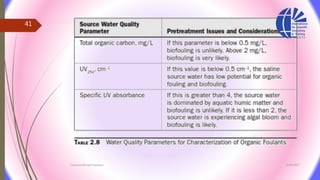
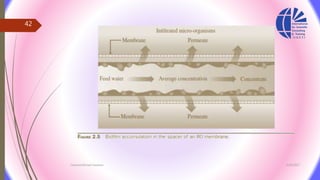
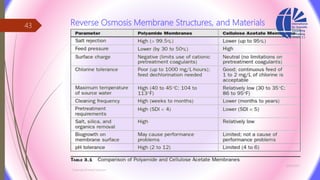
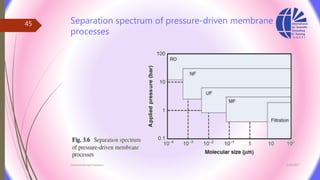
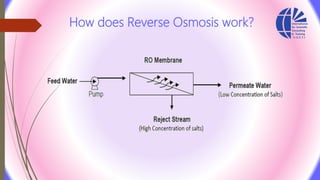
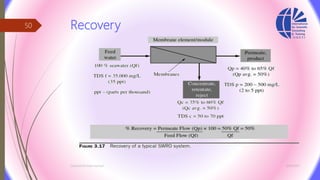
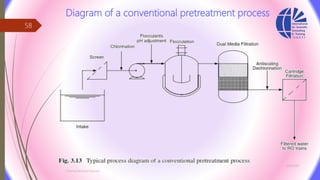
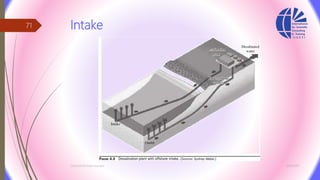
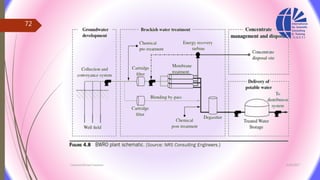
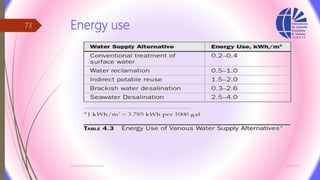
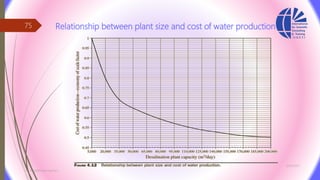
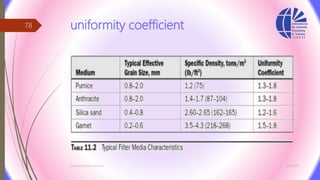
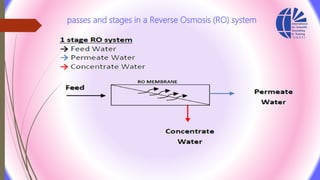
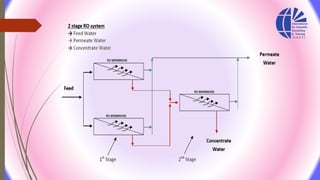
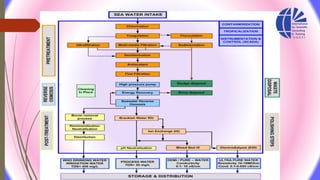
The document discusses various water desalination technologies, including thermal distillation, reverse osmosis, electrodialysis, and ion exchange. It provides detailed insights into the mechanisms, applications, and efficiency of these processes in producing potable water from saline sources, as well as challenges and factors affecting water quality. The text emphasizes the importance of pretreatment and post-treatment processes in ensuring the quality of the desalinated water.