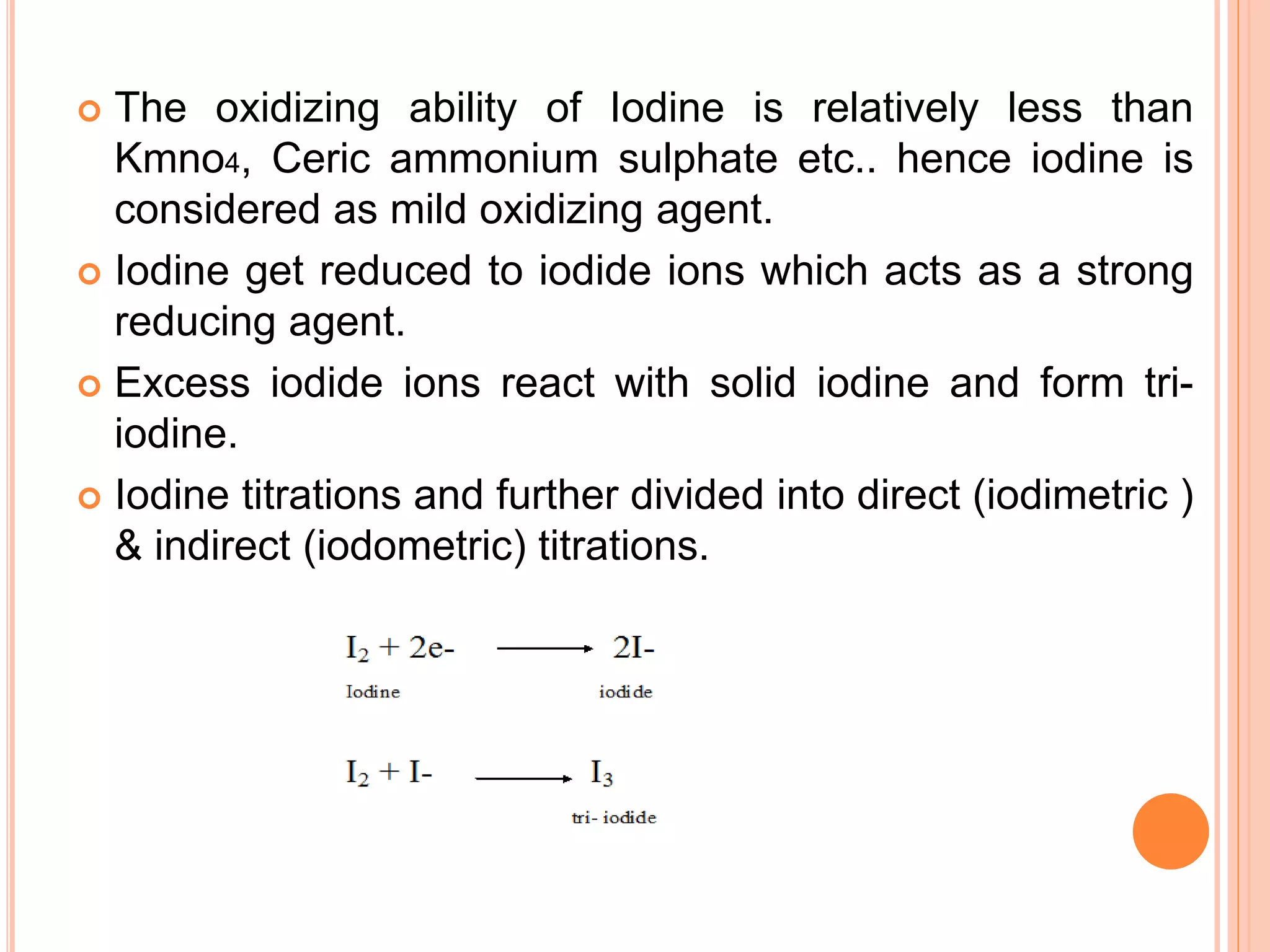
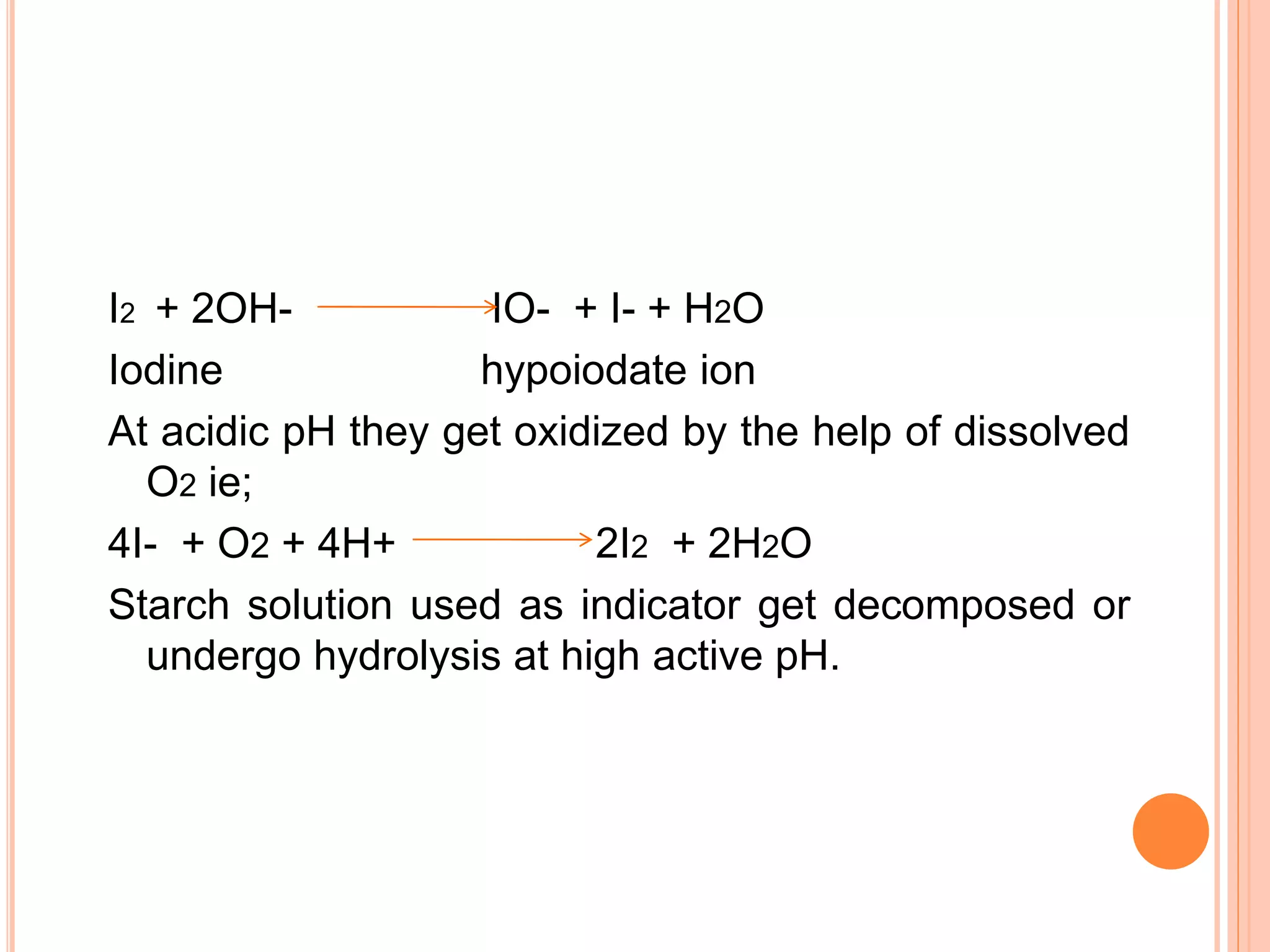
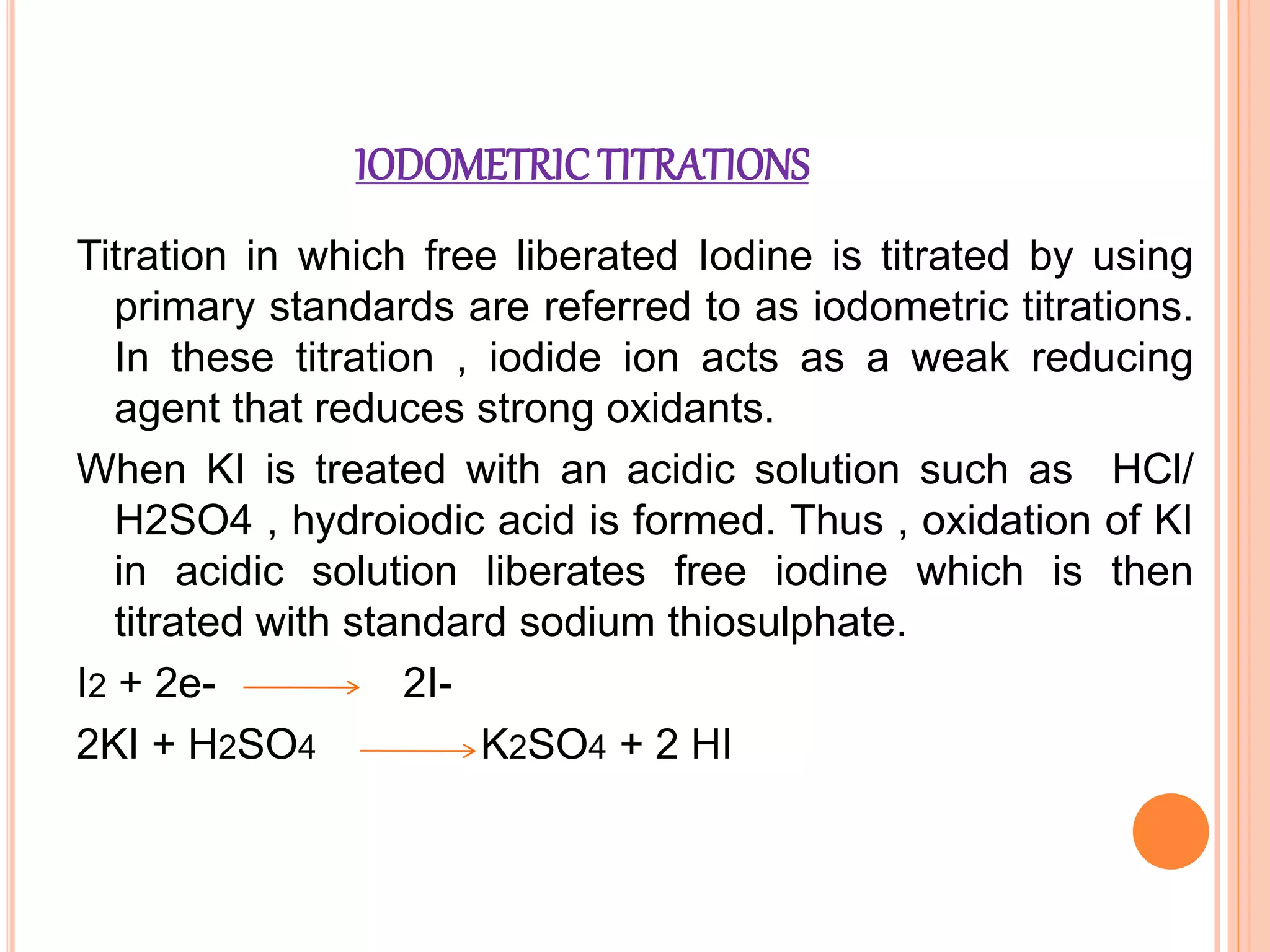
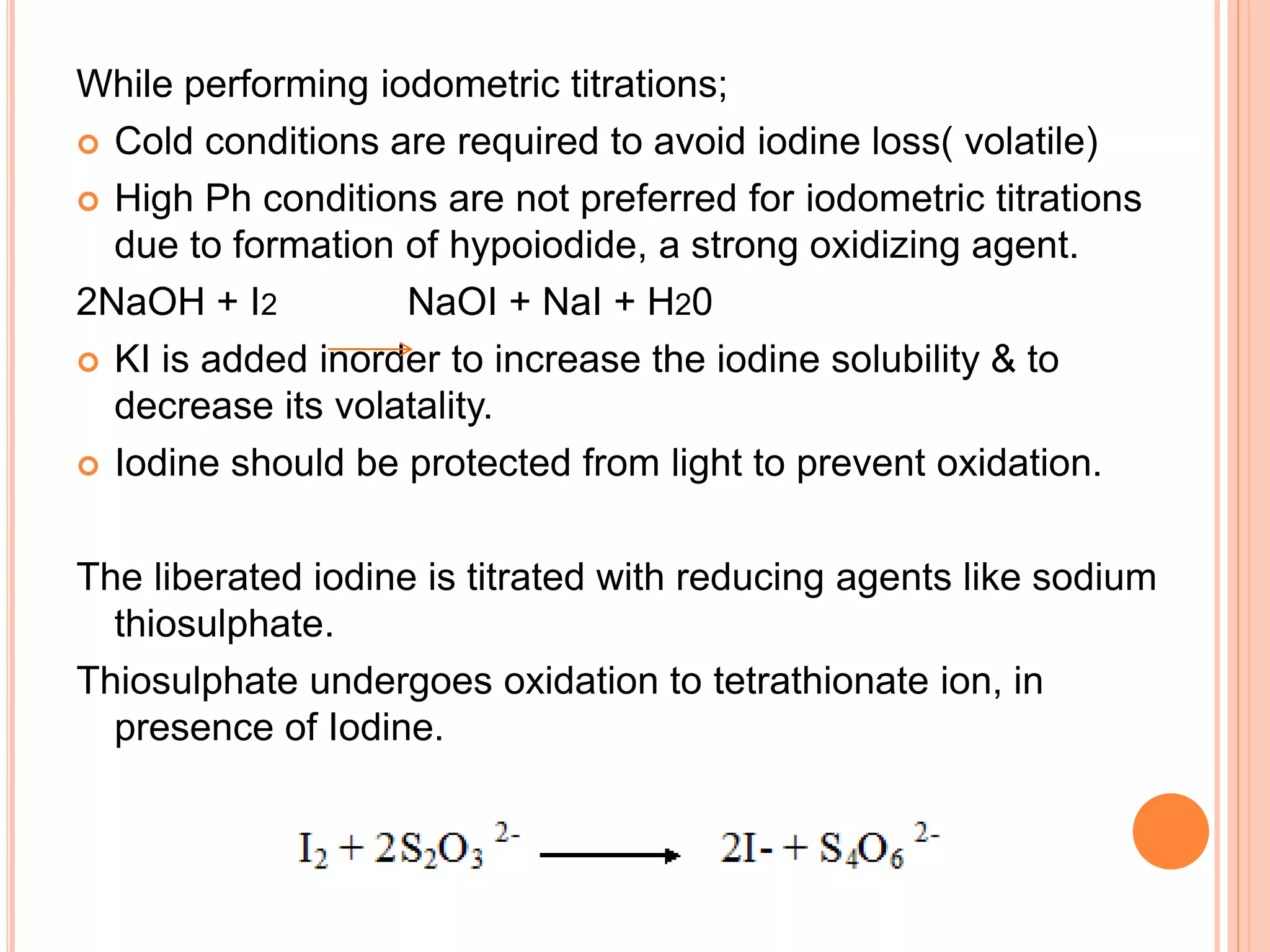
This document discusses iodine titrations, including iodimetric and iodometric titrations. Iodimetric titrations use iodine as the oxidizing agent, which reacts with strong reductants. Iodometric titrations involve liberating iodine from potassium iodide using an acid, then titrating the free iodine with sodium thiosulfate. Starch is commonly used as an indicator, turning blue when iodine is present. The document also provides procedures for preparing standard iodine and sodium thiosulfate solutions and describes how to standardize them using arsenic trioxide or potassium iodate, respectively.