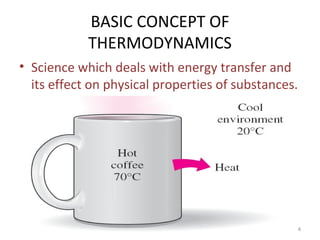
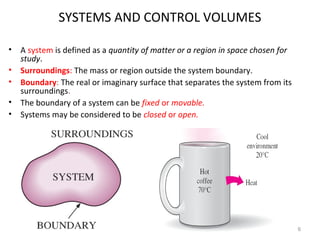
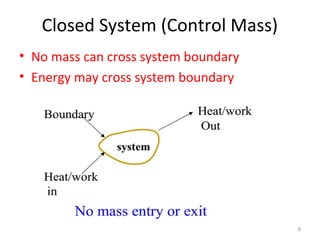
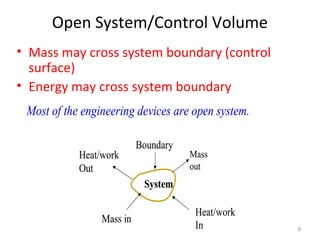
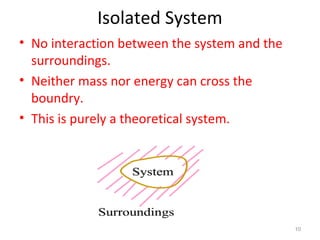
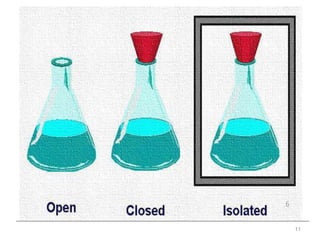
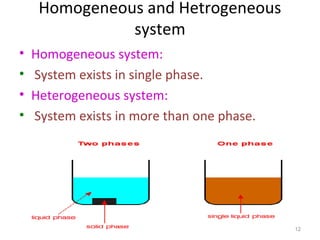
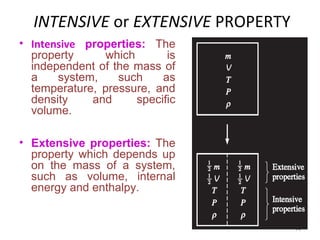
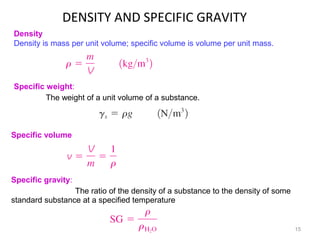
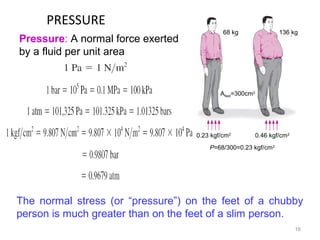
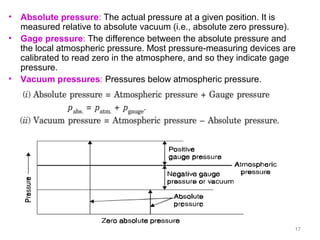
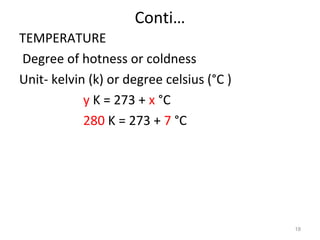
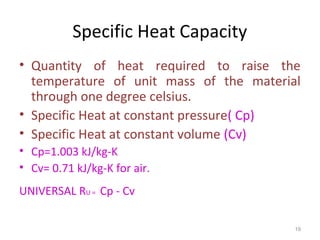
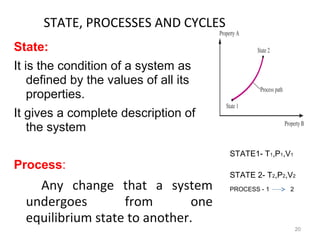
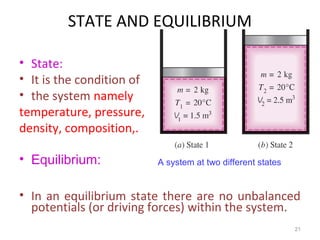
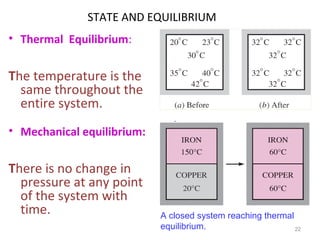
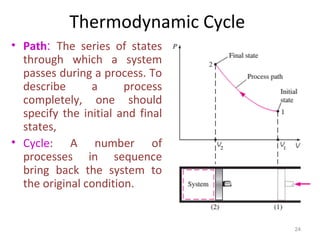
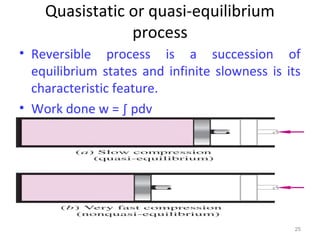
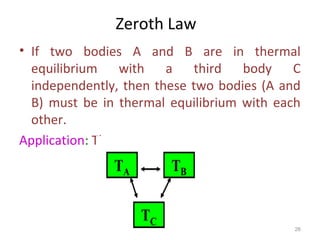
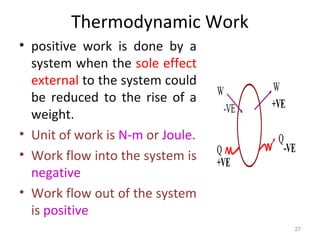
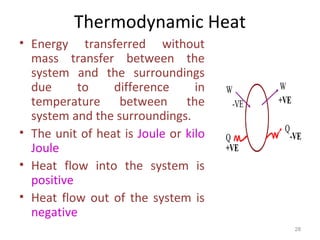
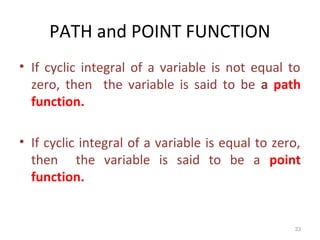
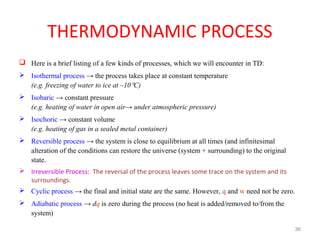
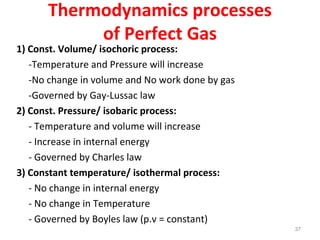
This document provides an introduction to engineering thermodynamics. It defines key concepts like systems, properties, processes, and the first law of thermodynamics. Specific topics covered include the classification of thermodynamic systems as closed or open, homogeneous or heterogeneous. The document also discusses intensive and extensive properties, pressure, temperature, and the gas laws of Boyle, Charles, and Gay-Lussac. Common thermodynamic processes like isothermal, isobaric, isochoric, and adiabatic processes are defined. The first law of thermodynamics relating heat, work, and changes in internal energy is stated.