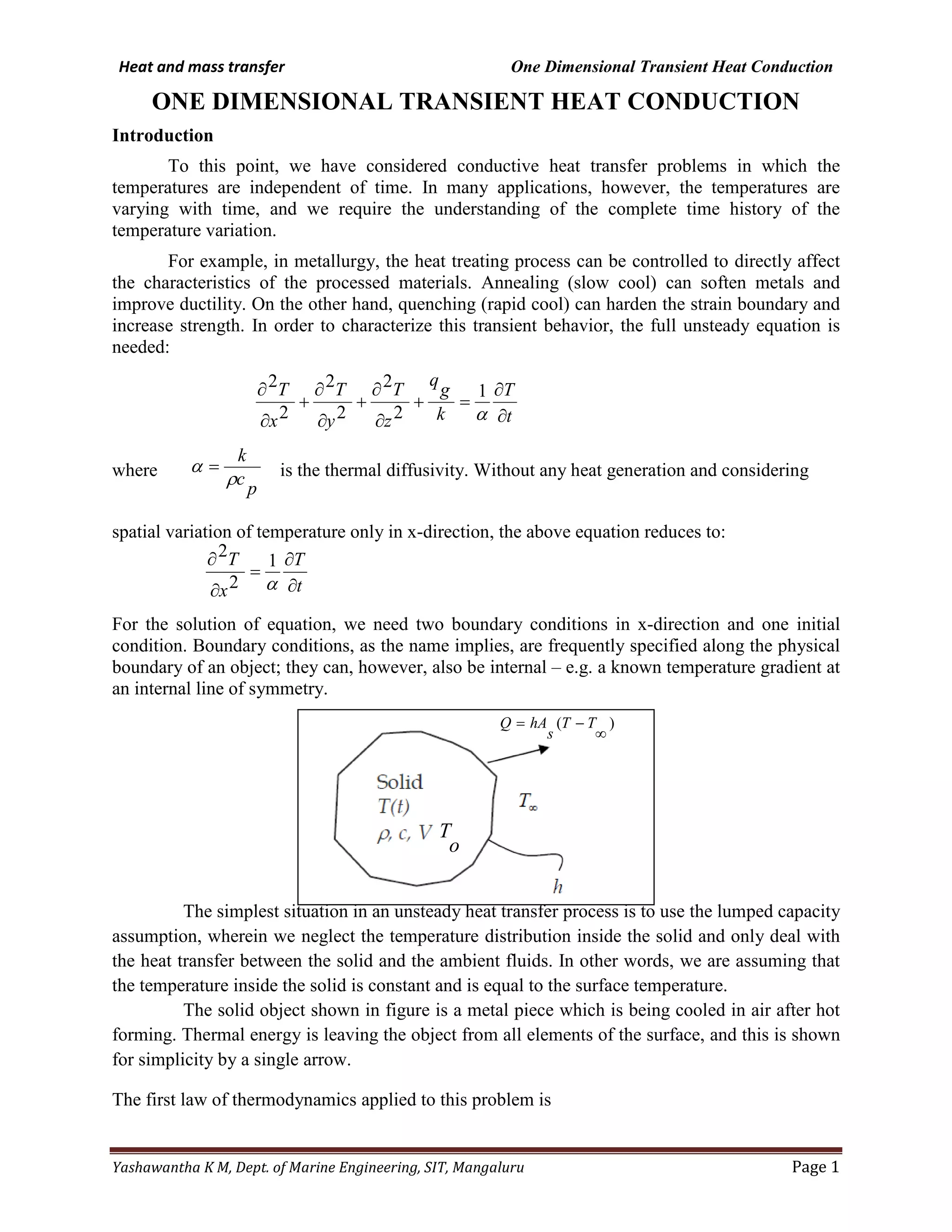
This document discusses one dimensional transient heat conduction. It introduces the full unsteady heat conduction equation and describes how it can be used to model time-varying temperature changes, as in heat treating processes. The lumped capacity assumption is described, where the temperature inside a solid is assumed constant and equal to the surface temperature. An example problem is presented of a metal piece cooling in air after forming. The general solution of the ordinary differential equation for this problem is given. Dimensionless numbers like the Biot number and Fourier number are also defined, with the Biot number representing the ratio of external to internal heat transfer resistance.
![Heat and mass transfer One Dimensional Transient Heat Conduction
Yashawantha K M, Dept. of Marine Engineering, SIT, Mangaluru Page 2
[Heat out of the object during time dt] = [decrease of internal thermal energy of
object during time dt]
Let consider that a solid f arbitrary shape volume v, total surface area As, thermal conductivity ks,
density ρ , specific heat c. uniform temperature To is suddenly immersed at the time t=0 in a well stirred
fluid which is kept at a uniform temperature
∞
T as shown in figure
The above equation is an ordinal differential equation for the temperature θ (t) and its
general solution given as,
By boundary condition,
(Refer HMTDB Page No 58)
dt
dT
VcTTh
s
A ρ=−
∞
)(
dt
dT
TT
Vc
h
s
A
=−
∞
)(
ρ
)( TT
Vc
h
s
A
dt
dT
−
∞
−=
ρ
)(
∞
−= TT
Vc
h
s
A
dt
dT
ρ
0)( =
∞
−− TT
Vc
h
s
A
dt
dT
ρ
Vc
h
s
A
m
ρ
=
0)( =
∞
−− TTm
dt
dT θ=
∞
−TT
dt
d
dt
dT θ
=0=− θ
θ
m
dt
d
mtCet −=)(θ
0==
∞
− tat
o
T
o
T θ
Co=θ
mtet o
−=θθ )(
mte
o
t −=
θ
θ )(
mte
T
o
T
TT
−=
∞
−
∞
−](https://image.slidesharecdn.com/unit3transientheatcondution-150410130500-conversion-gate01/75/Unit-3-transient-heat-condution-2-2048.jpg)

