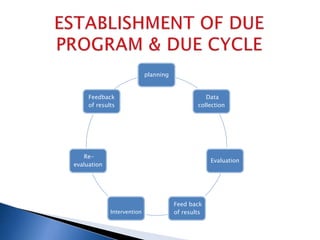
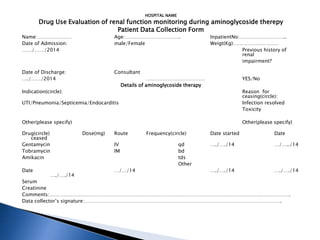
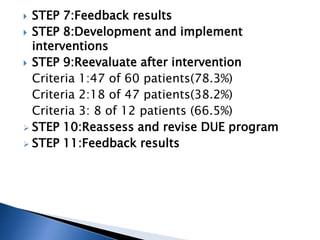
Drug use evaluation (DUE) is a quality improvement process that reviews prescribing patterns to promote appropriate drug use. It involves identifying a drug or therapeutic area, developing criteria and standards, collecting data, evaluating results, providing feedback, and implementing interventions. The process then reevaluates drug use and revises the DUE program as needed. The presented document outlines the 11 steps of a DUE process focusing on monitoring renal function during aminoglycoside therapy.