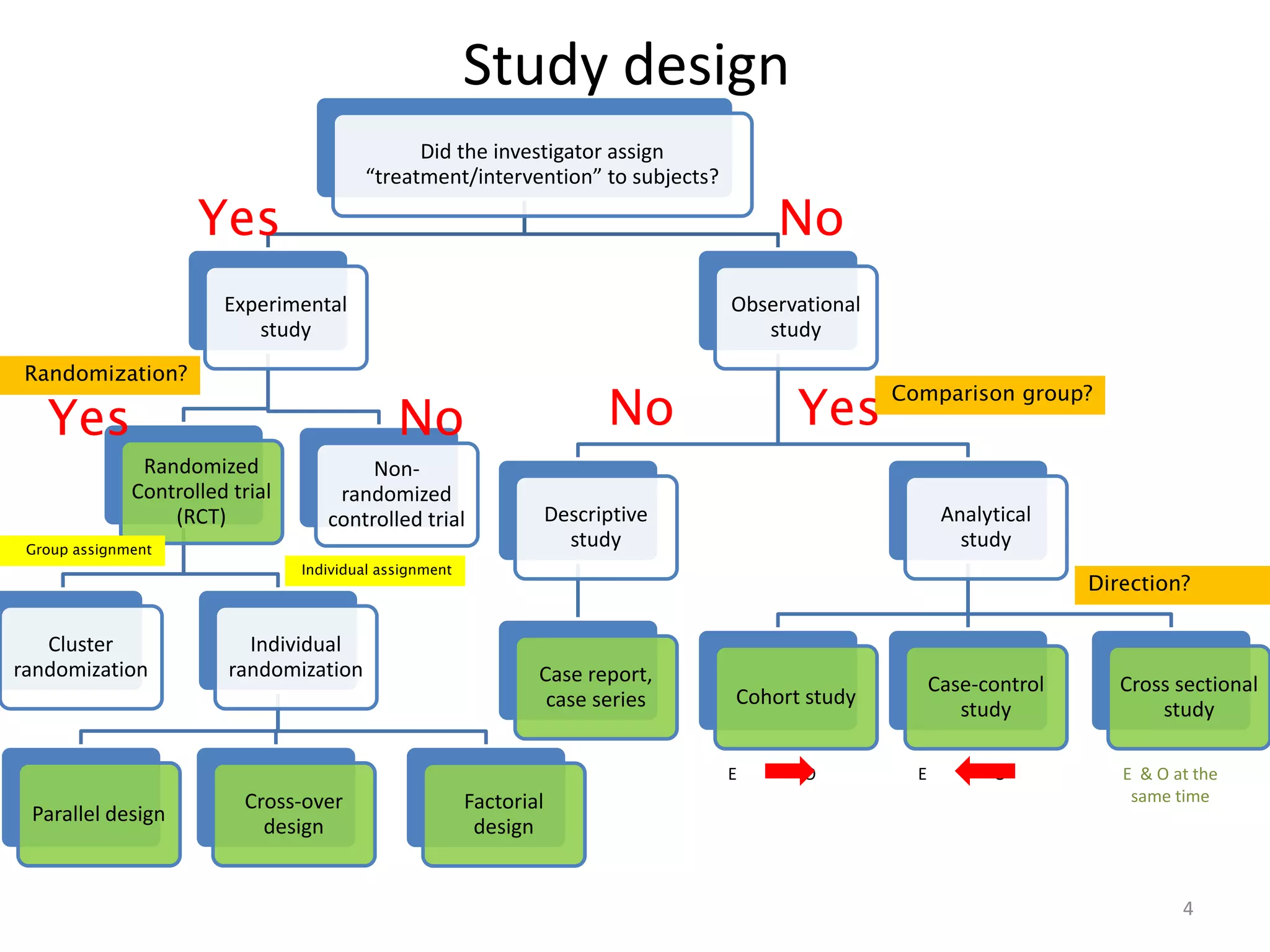
The document discusses various study designs used in pharmacoepidemiology research, including observational and experimental designs. Observational designs include case reports/series, ecological studies, cross-sectional studies, case-control studies, and cohort studies. Experimental designs include randomized clinical trials that can have parallel, crossover, or factorial designs. The document provides examples and compares the advantages and disadvantages of each design for investigating drug effects in human populations.