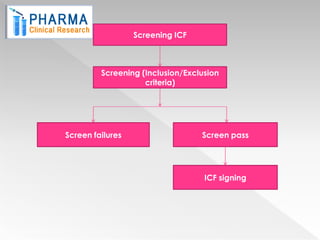
The document outlines the patient enrolment process essential for conducting clinical trials, highlighting the importance of informed consent and the steps involved, such as pre-screening, counseling, and randomization. It emphasizes the need for detailed patient education regarding the trial and addresses the patient's autonomy in participating after receiving adequate information. The process ensures that only eligible subjects, based on inclusion/exclusion criteria, are enrolled in the study.