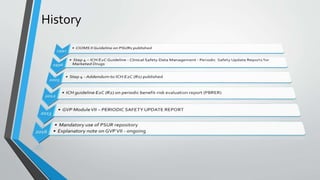
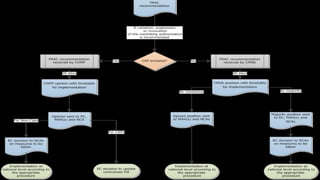
Periodic Safety Update Reports (PSURs) are documents submitted by marketing authorization holders to provide an evaluation of a medicine's risk-benefit balance at defined intervals during the post-authorization phase. PSURs must be submitted within 70 or 90 calendar days depending on the interval and contain information on emerging safety issues, benefit-risk analyses, risk management plans, and sources of safety data. The European Medicines Agency publishes the European Union Reference Dates list which mandates the submission timelines and frequencies for PSURs of specific medicines. A single assessment of PSURs is conducted for medicines with the same active substance.