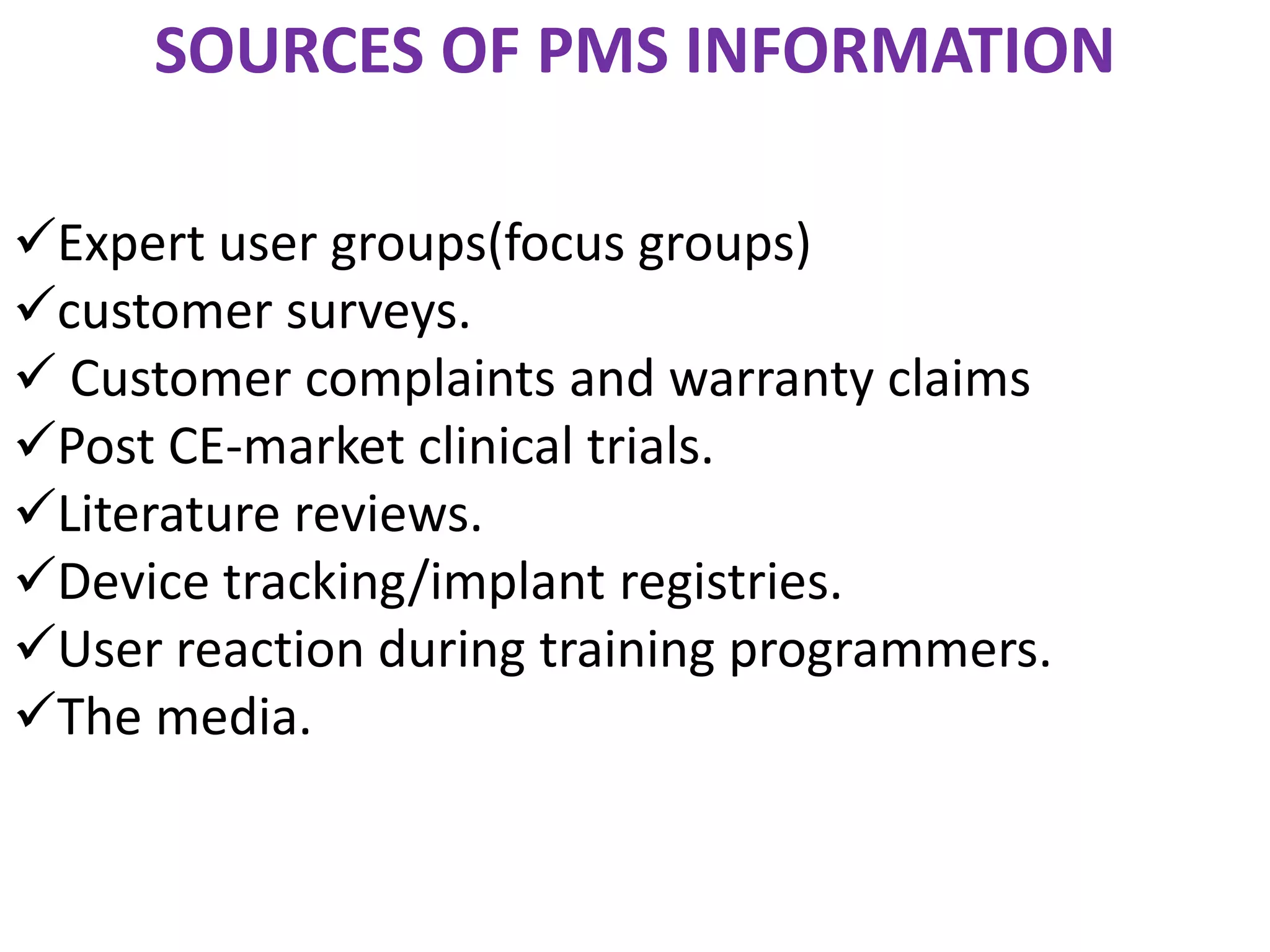
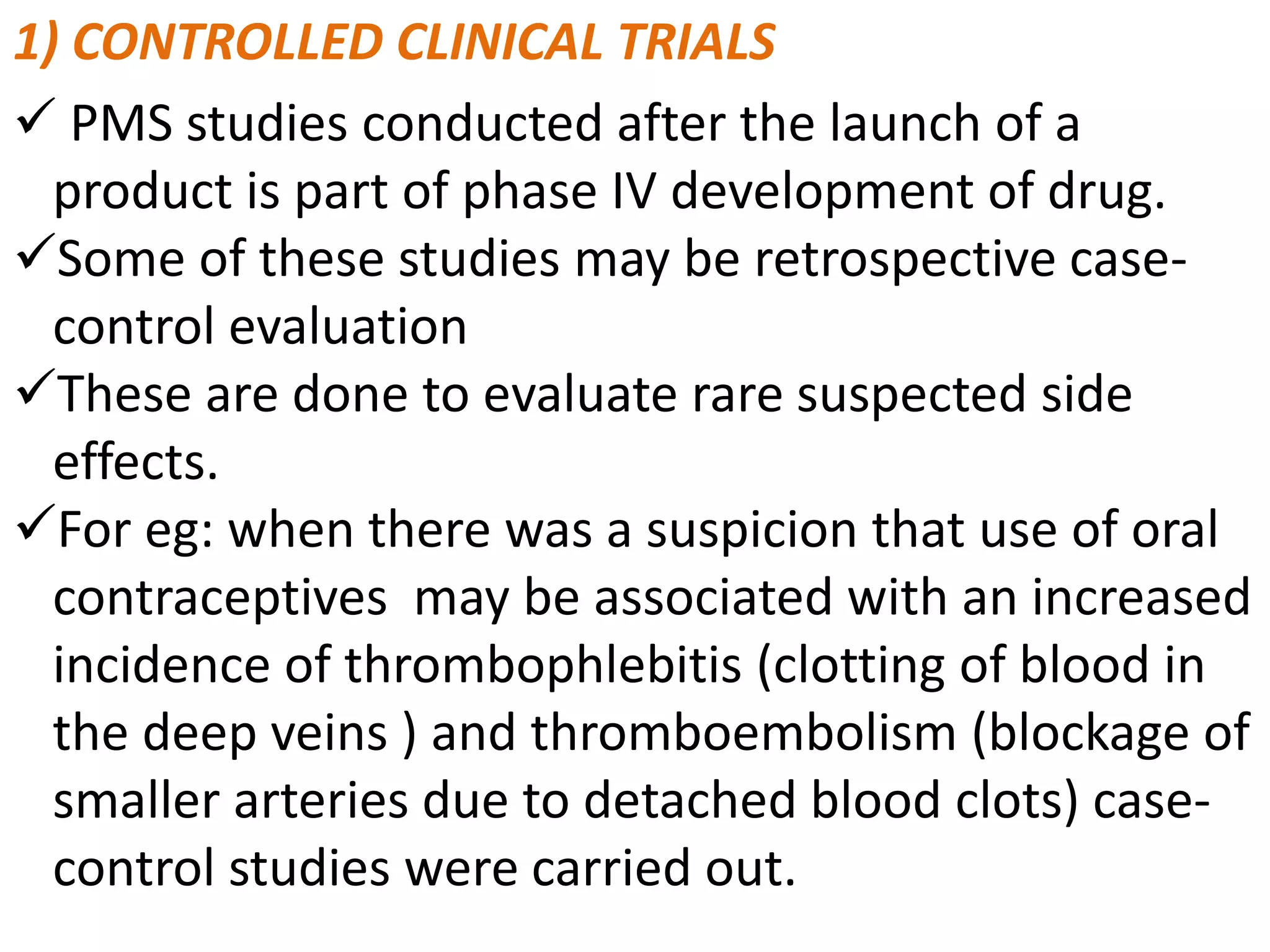
Post-marketing surveillance (PMS) monitors drug and medical device safety after market release using approaches like spontaneous reporting databases, prescription monitoring, and health records. PMS identifies potential safety issues through data review and helps detect rare or long-term adverse effects not seen in pre-market clinical trials which have limited patient populations and durations. PMS provides additional safety and efficacy information on marketed products and allows monitoring of special patient groups. Common PMS methods include spontaneous reporting, observational studies, randomized trials, and active surveillance networks.