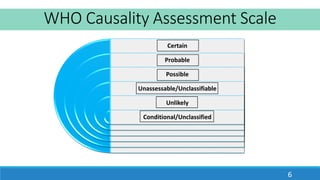
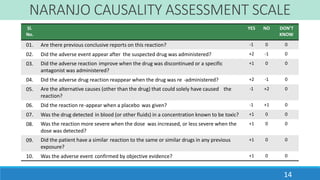
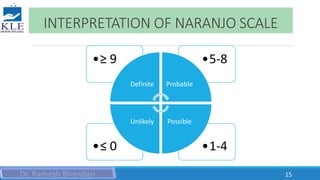
This document discusses different methods for assessing the causality of adverse drug reactions (ADRs). It describes three main types of methods: 1) Expert opinion or clinical judgment methods like the WHO causality assessment scale, 2) Algorithmic methods like the Naranjo scale that use questionnaires and scoring systems, 3) Probabilistic or Bayesian methods that calculate the probability of drug causation based on prior probabilities. The WHO scale and Naranjo scale are discussed in detail as examples of each method.