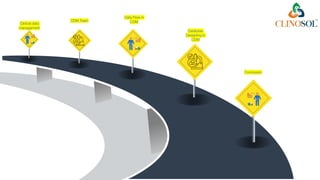
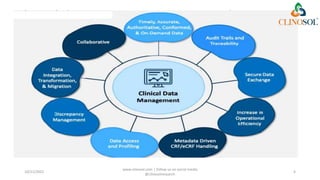
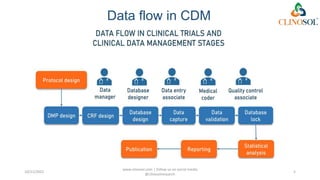
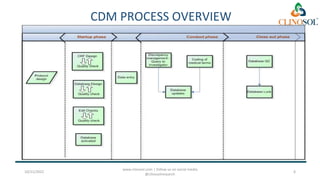
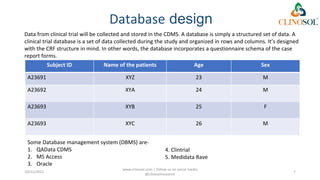
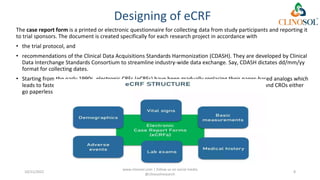
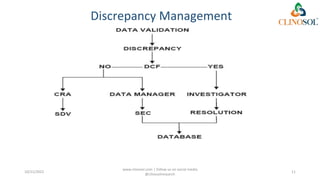
The document discusses clinical data management (CDM), detailing its processes, roles, and the transition from paper-based to electronic data systems in clinical trials. It outlines the responsibilities of the CDM team, including data collection, validation, and locking of databases, as well as the use of case report forms. Overall, it emphasizes the importance of high-quality data for efficient drug development and regulatory compliance.