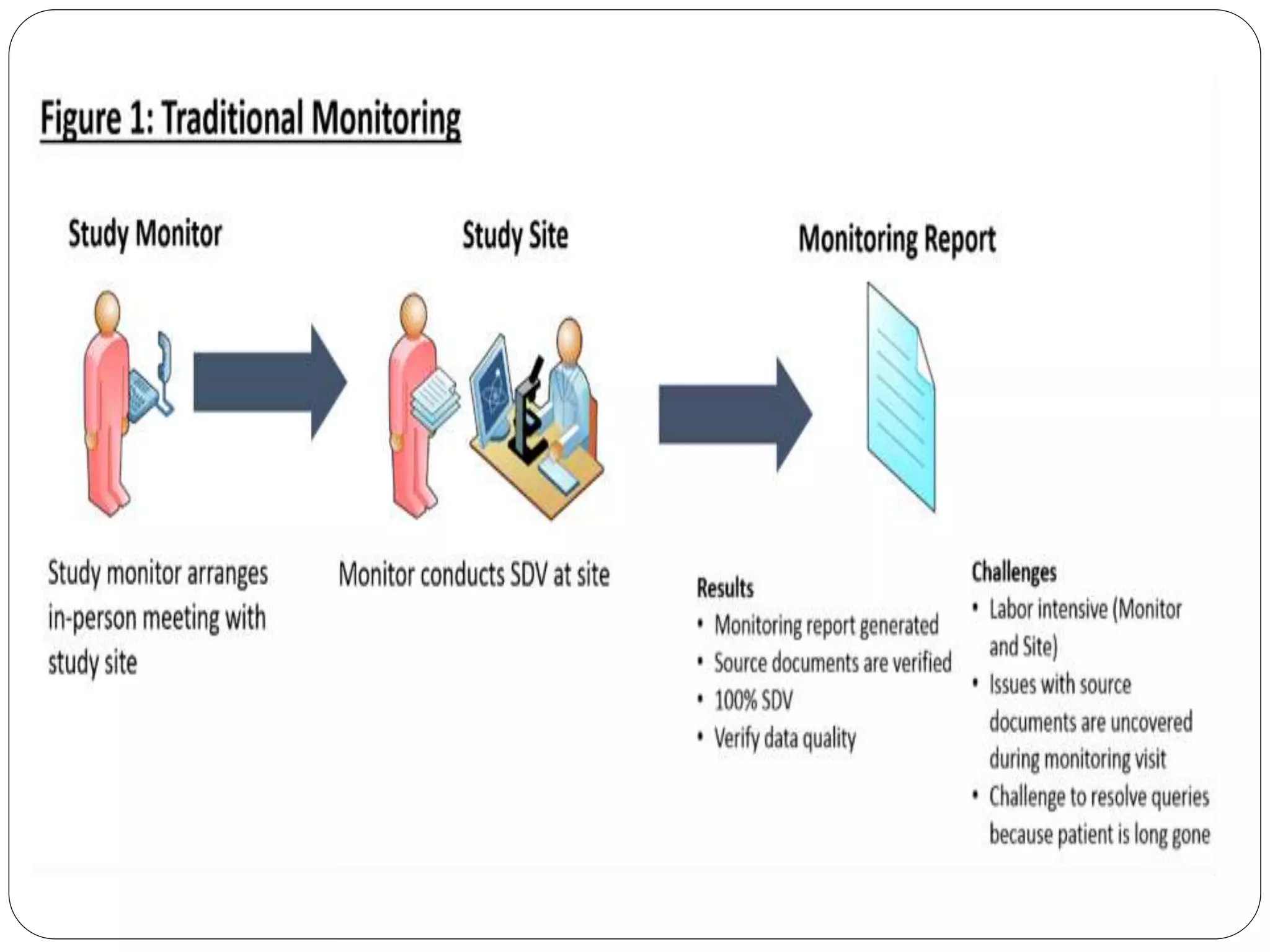
This document discusses clinical trial monitoring. It defines monitoring as overseeing a clinical trial to ensure it follows the protocol, GCP standards, and regulations. The purpose is to protect subject rights and ensure compliance and data quality. Factors like the protocol complexity, site enrollment rate, and performance determine monitoring frequency. Sites are monitored regularly through visits to ensure proper conduct, documentation, and issue resolution. Monitors are trained and qualified by the sponsor to perform monitoring tasks and activities according to a plan.














![Aspects of monitoring
According to the U.S. Food and Drug Administration's
Center of Drug Evaluation and Research, the top five
deficiency categories for site inspections caught by clinical
monitors as reported in the 2001 Report to the Nation[4] are:
Failure to follow investigation protocol (the procedures and
treatment subjects must undergo, as well as the schedule of
assessments)
Failure to keep adequate and accurate records
Problems with the informed consent form
Failure to report adverse events
Failure to account for the disposition of study drugs](https://image.slidesharecdn.com/monitoringofclinicaltrials-210520091847/75/Monitoring-of-clinical-trials-16-2048.jpg)


