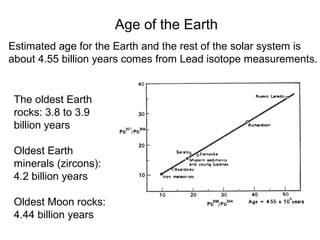
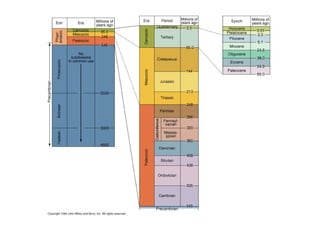
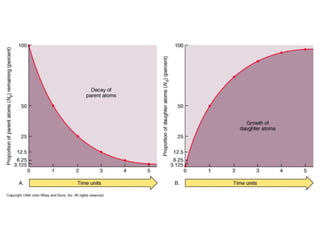
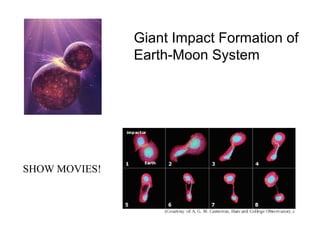
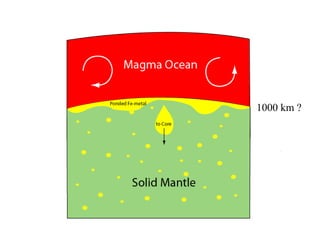
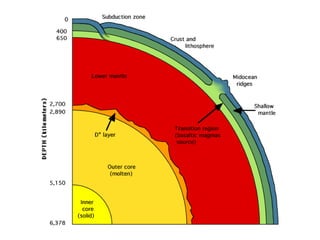
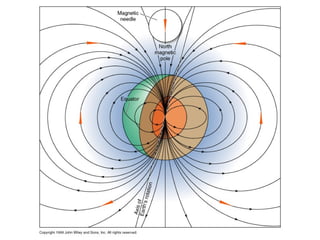
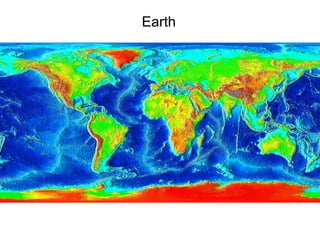
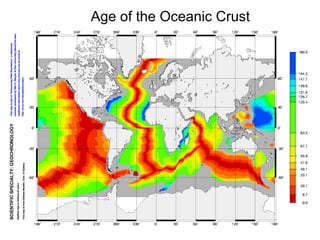
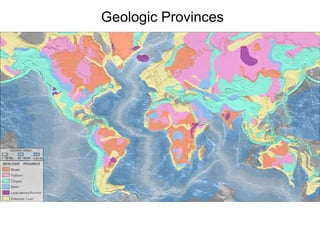
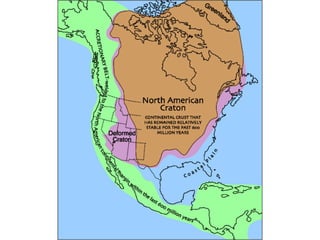
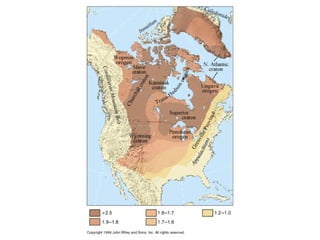
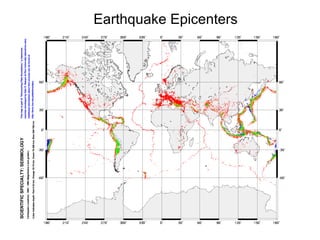
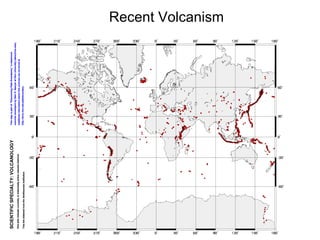
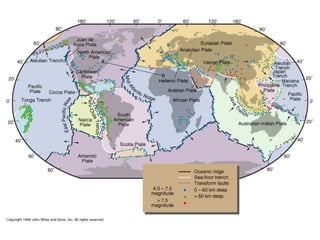
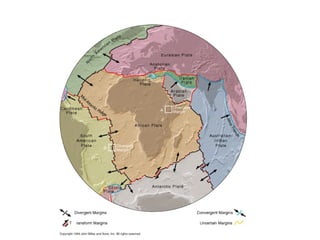
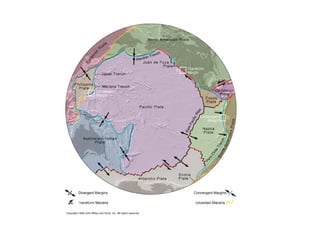
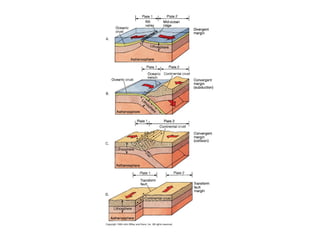
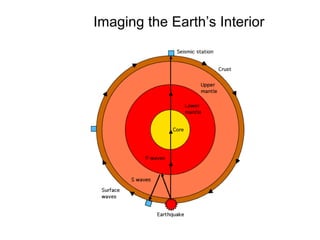
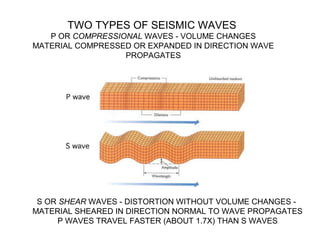
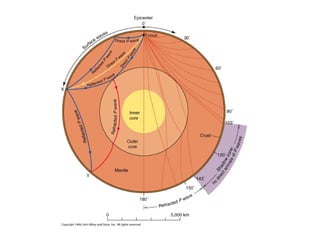
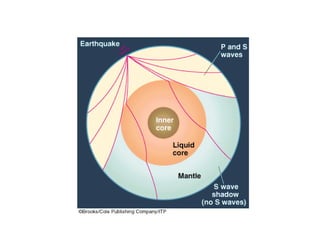
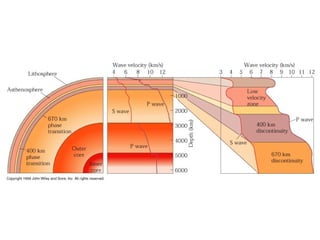
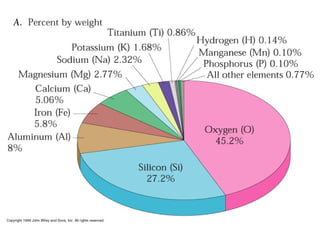
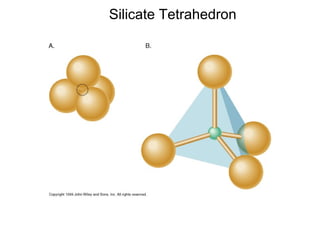
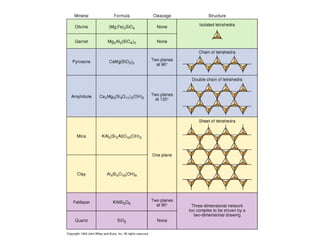
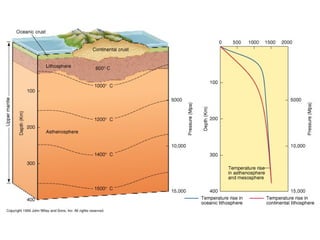
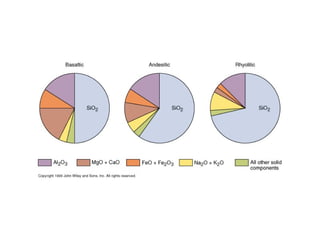
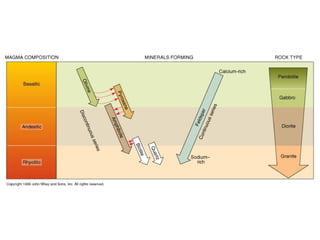
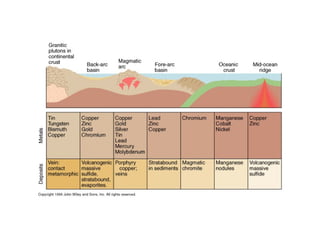
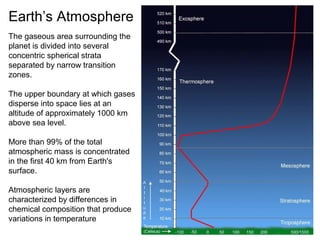
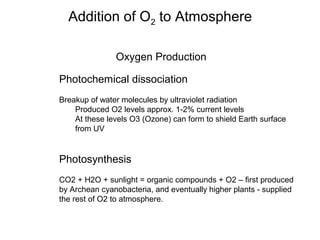
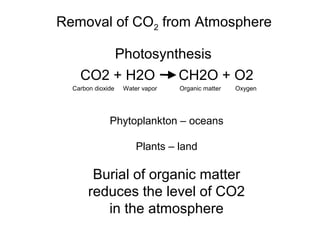
The document provides information about the Earth, including its formation approximately 4.5 billion years ago, composition and structure of its atmosphere, and how the atmosphere has changed over geological time through volcanic outgassing and the rise of oxygen through photosynthesis. It notes that the early Earth had a secondary atmosphere produced by volcanic outgassing rather than a primordial one, and that oxygen levels increased once cyanobacteria began producing oxygen through photosynthesis approximately 3.8 billion years ago.