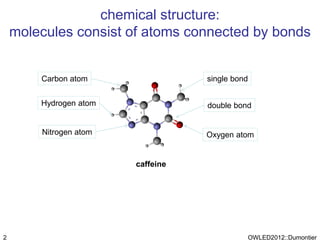
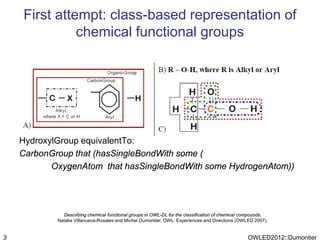
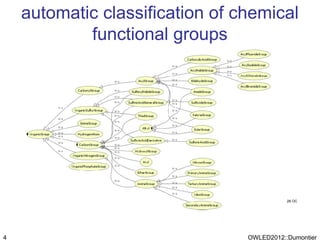
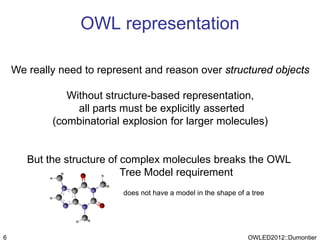
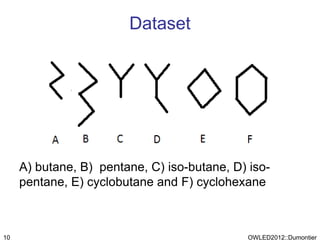
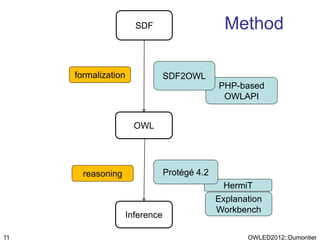
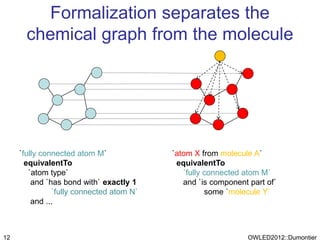
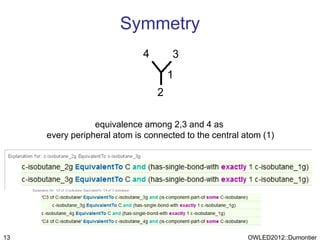
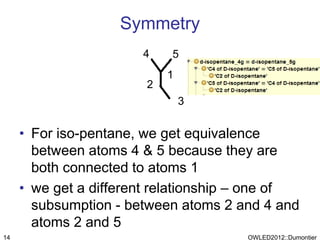
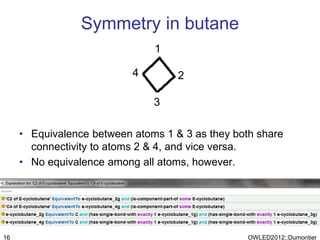
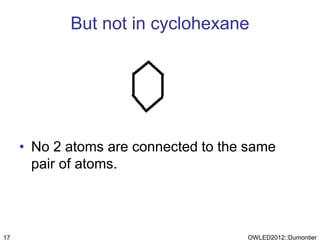
The document discusses the class-based representation of chemical structures using OWL-DL, focusing on the automatic classification of chemical functional groups and the challenges posed by complex molecular structures. It proposes the use of description graphs and rules to enhance OWL with capabilities for classifying chemical compounds, addressing issues like atomic connectivity and symmetry. The findings indicate that it is possible to represent and reason about molecular properties and equivalences within different molecules, paving the way for further research in chemical classification.