The document discusses key concepts in material technology including:
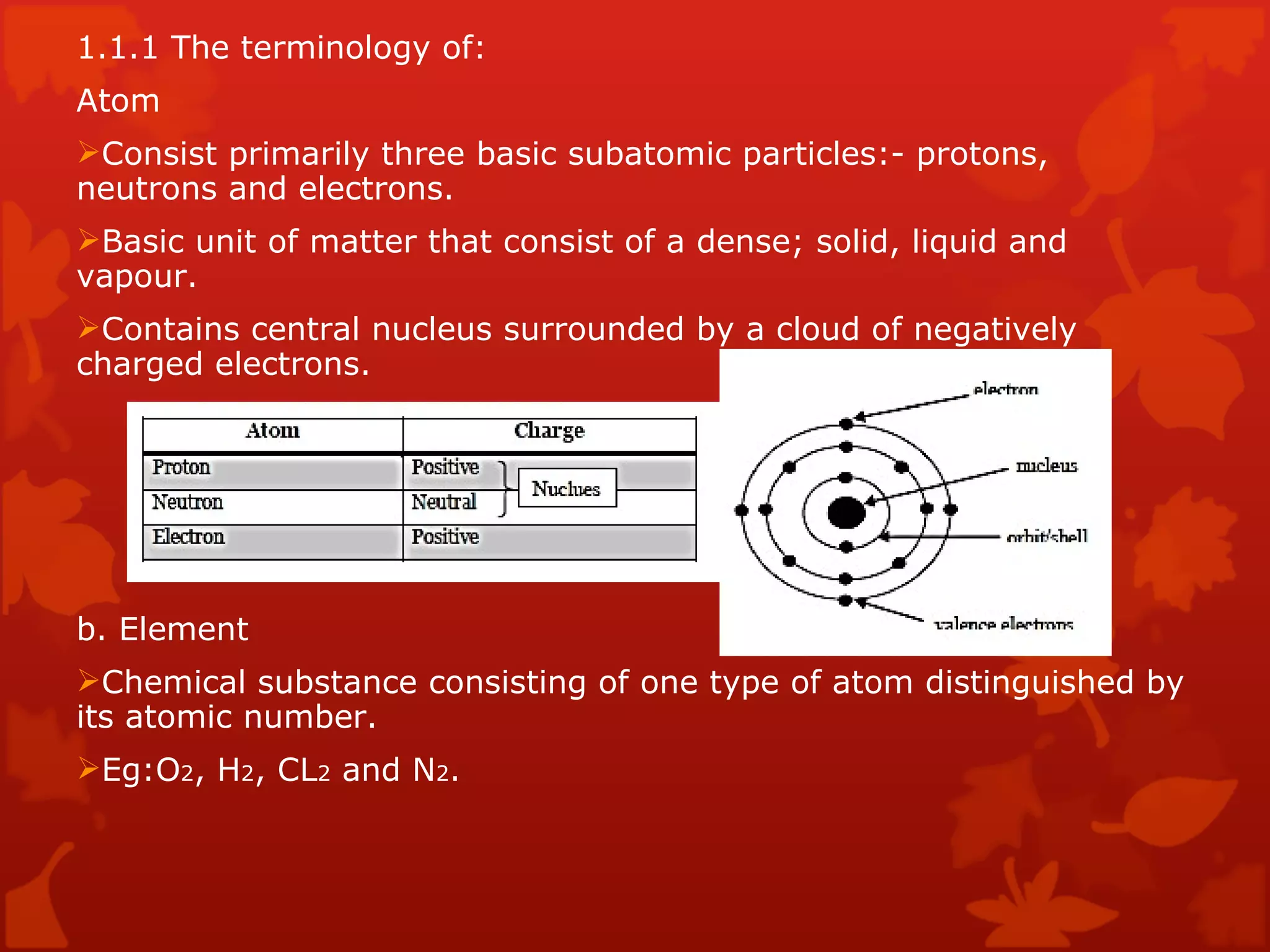
1. It defines the basic structure of atoms and different types of materials including elements, mixtures, and compounds.
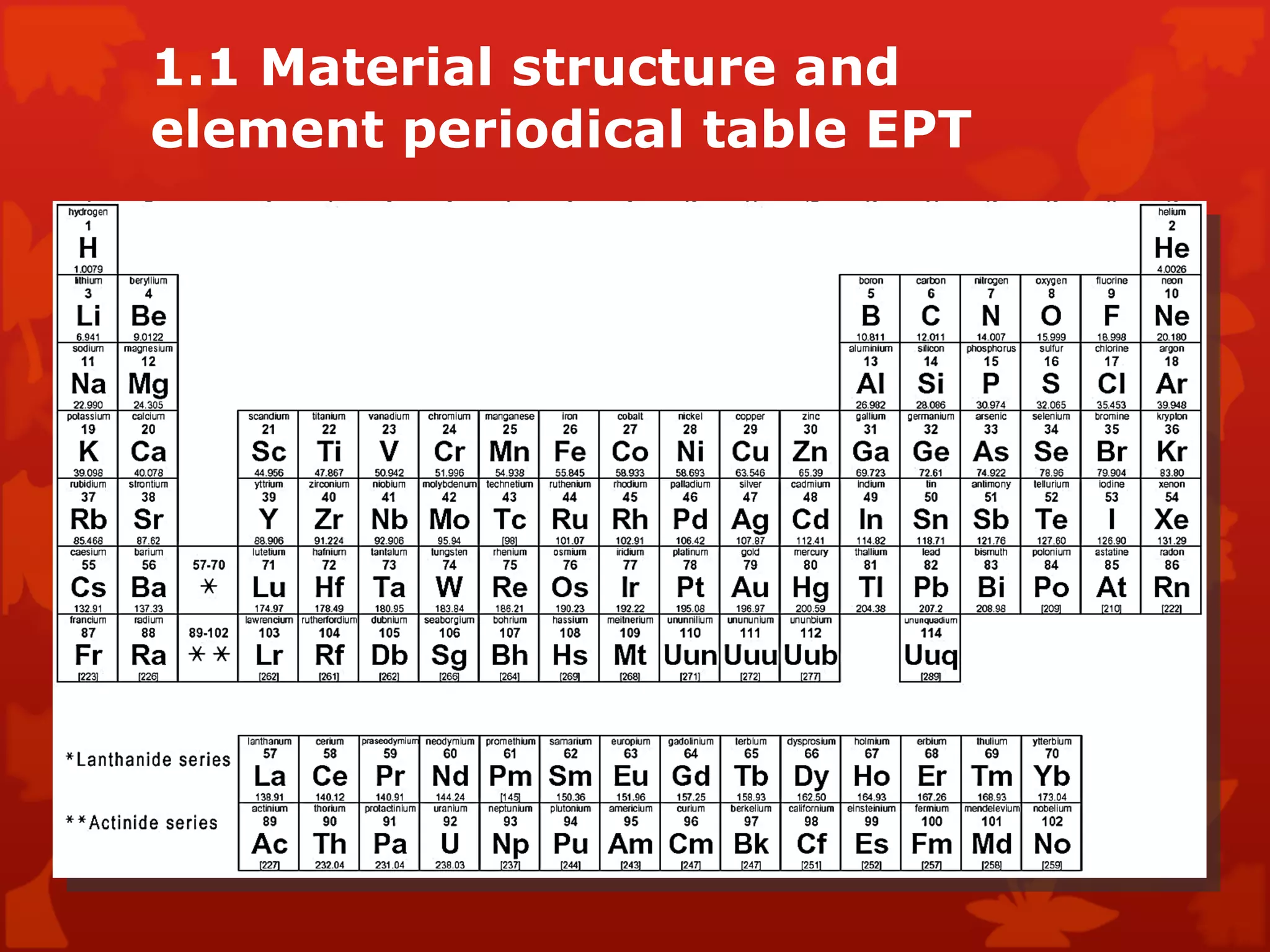
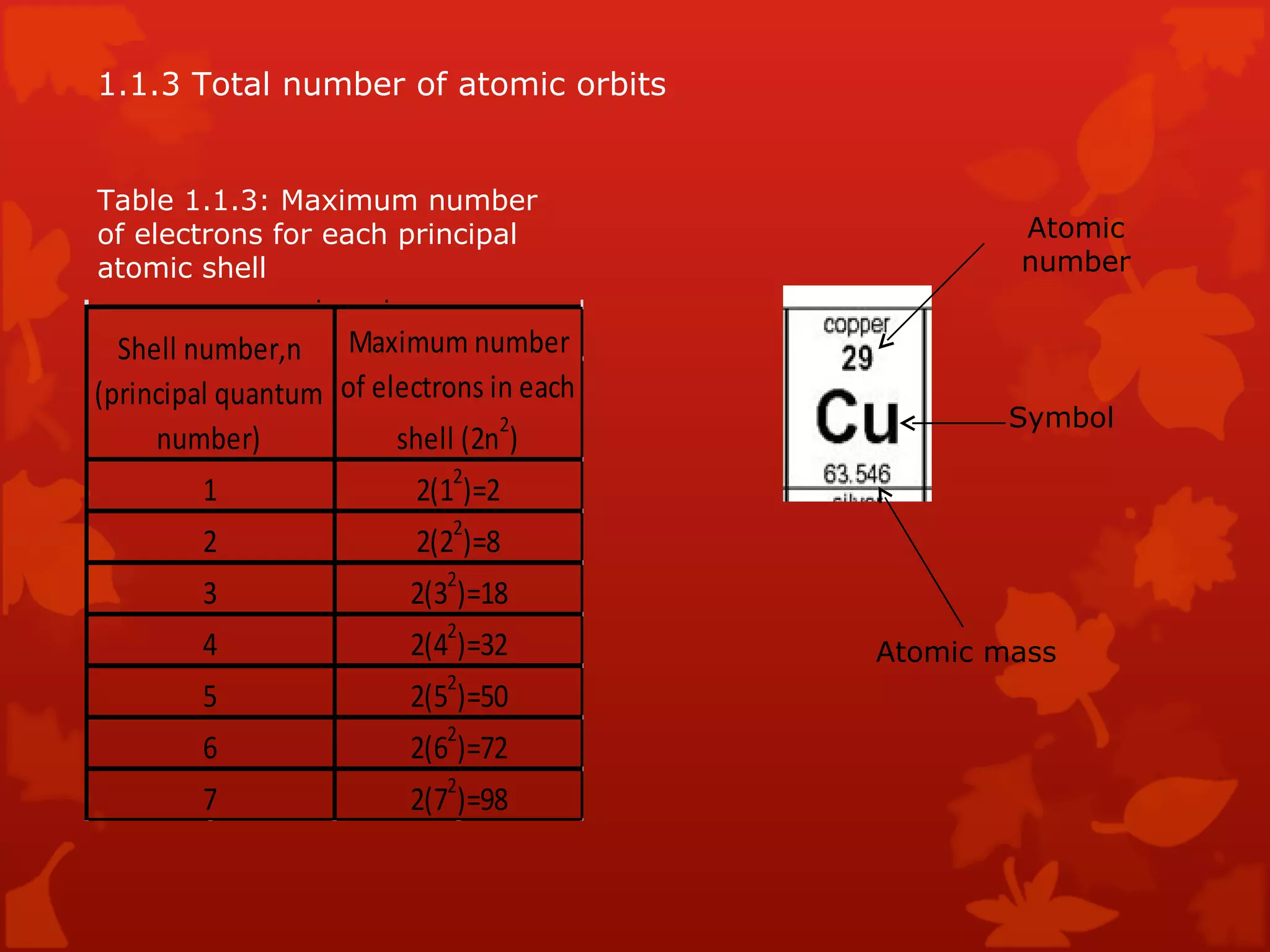
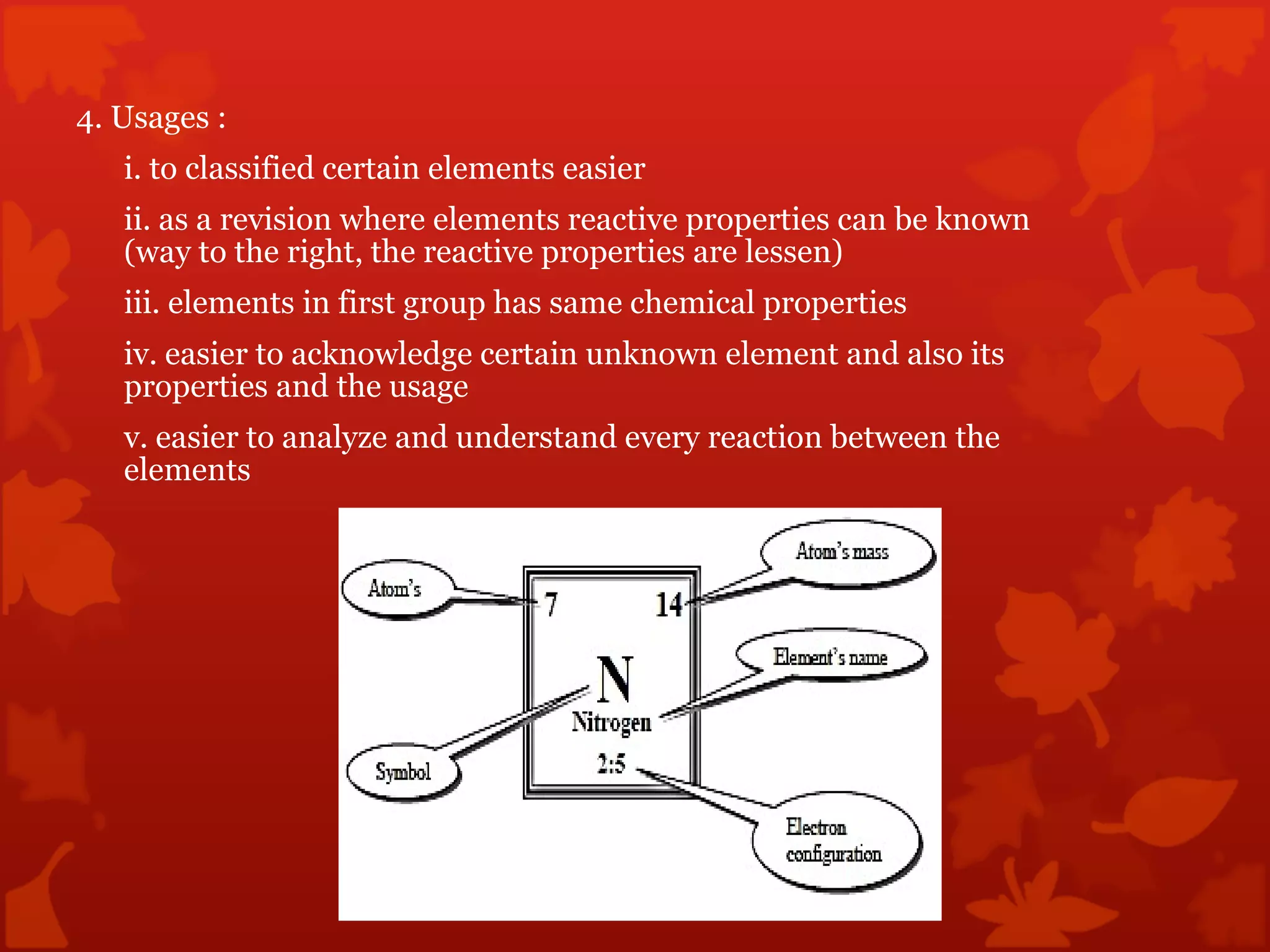
2. It describes atomic structure including atomic number, atomic mass, and atomic orbits. The periodic table is introduced as a way to classify and understand elements and their properties.
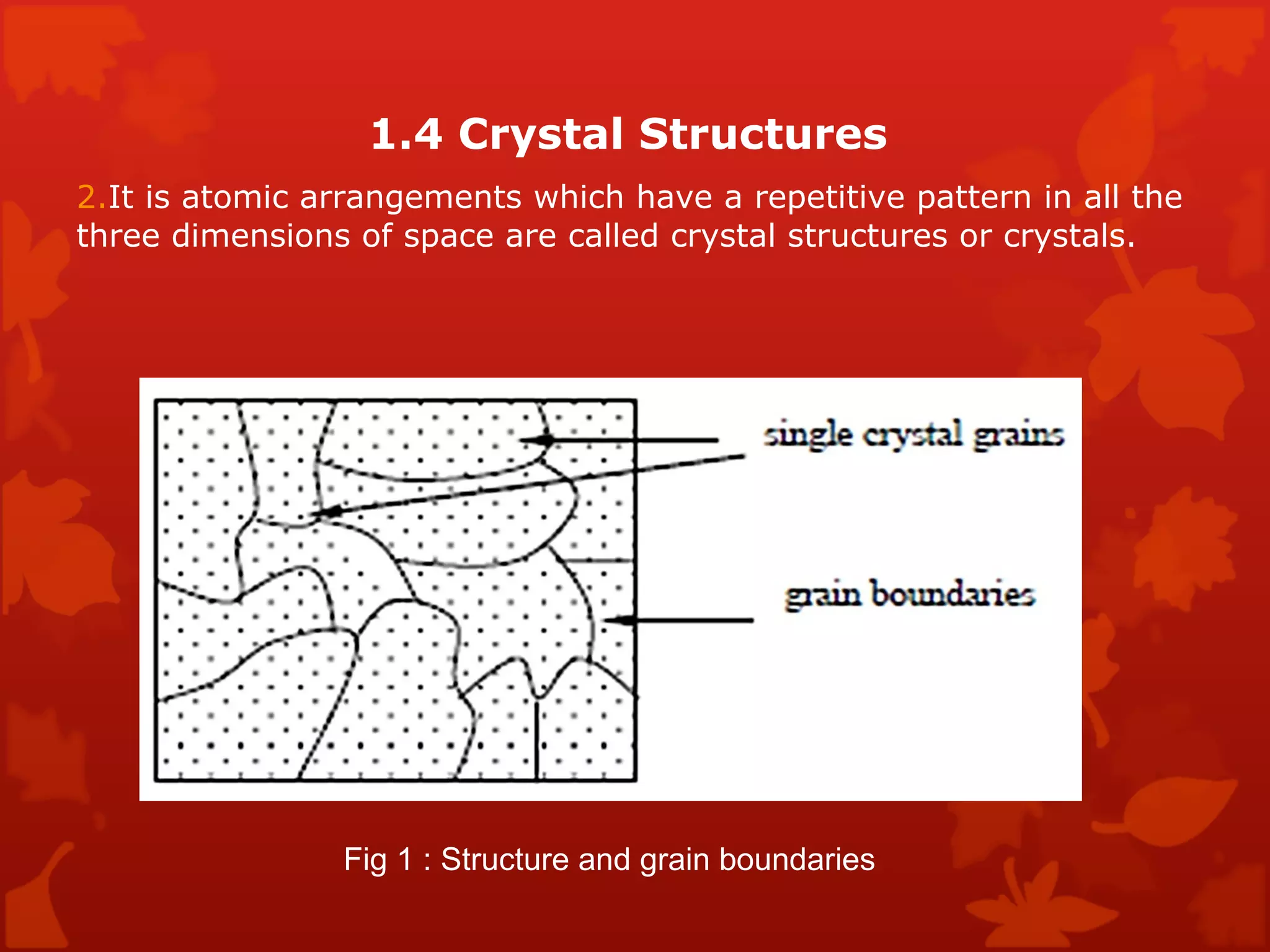
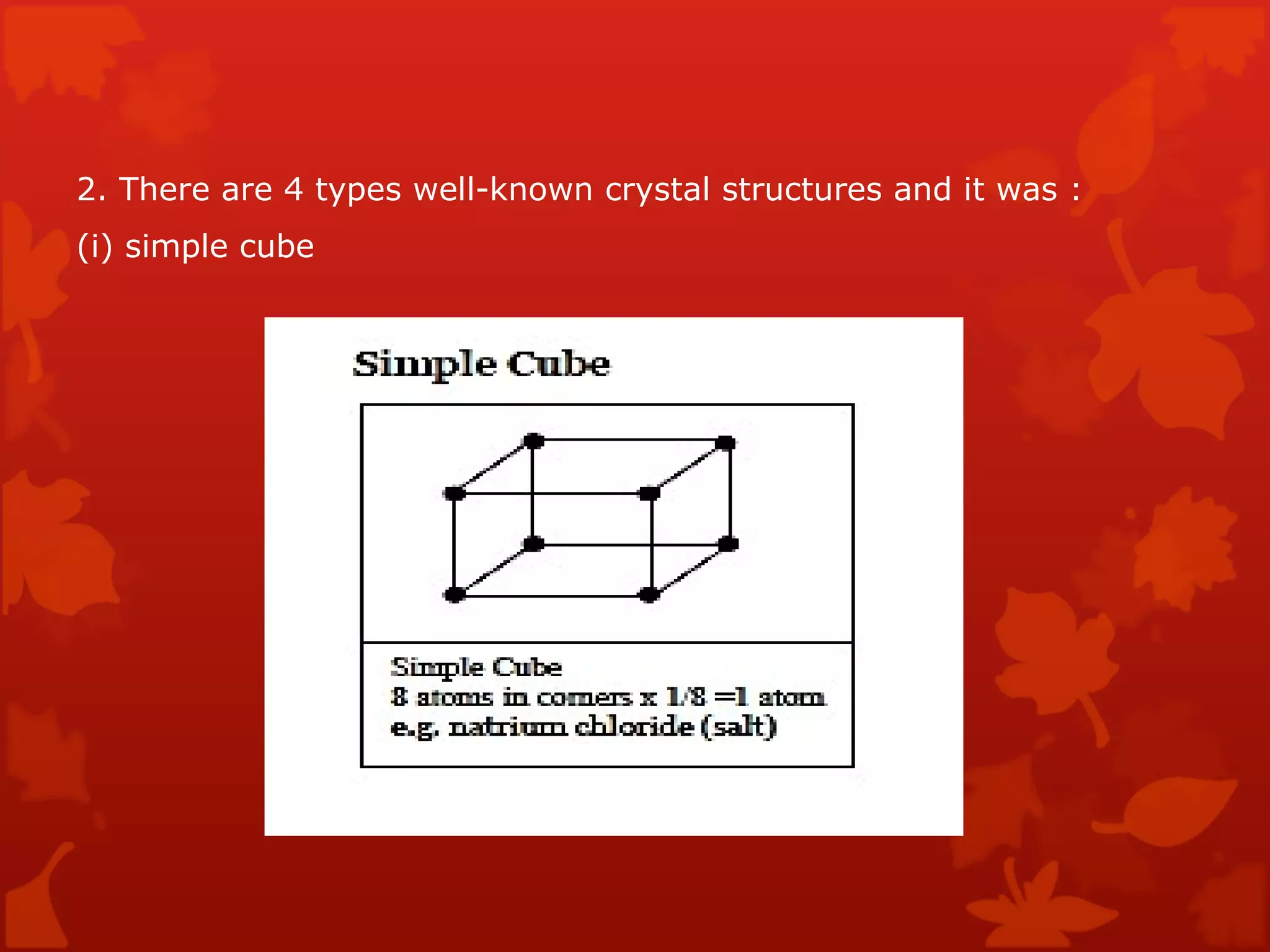
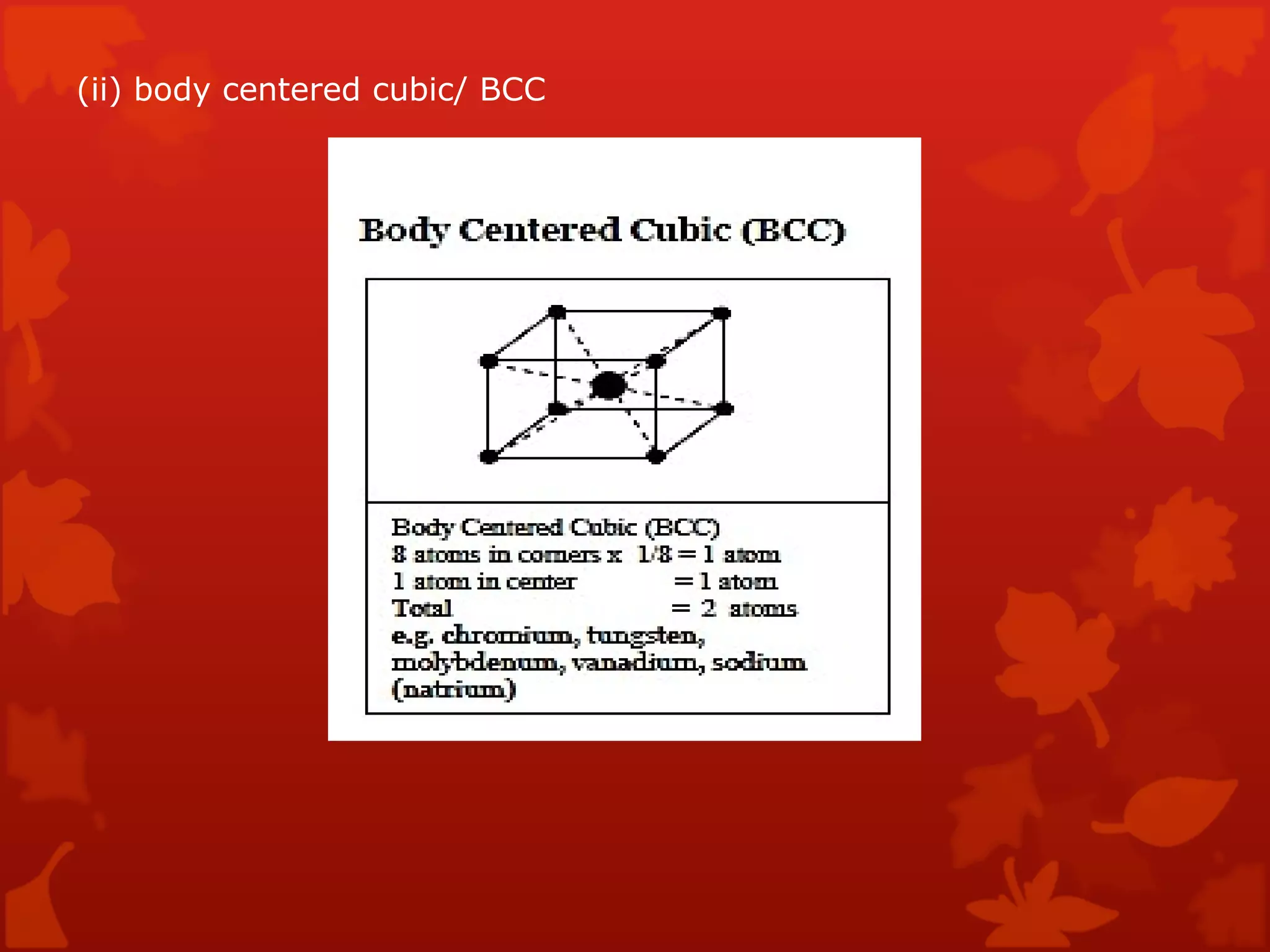
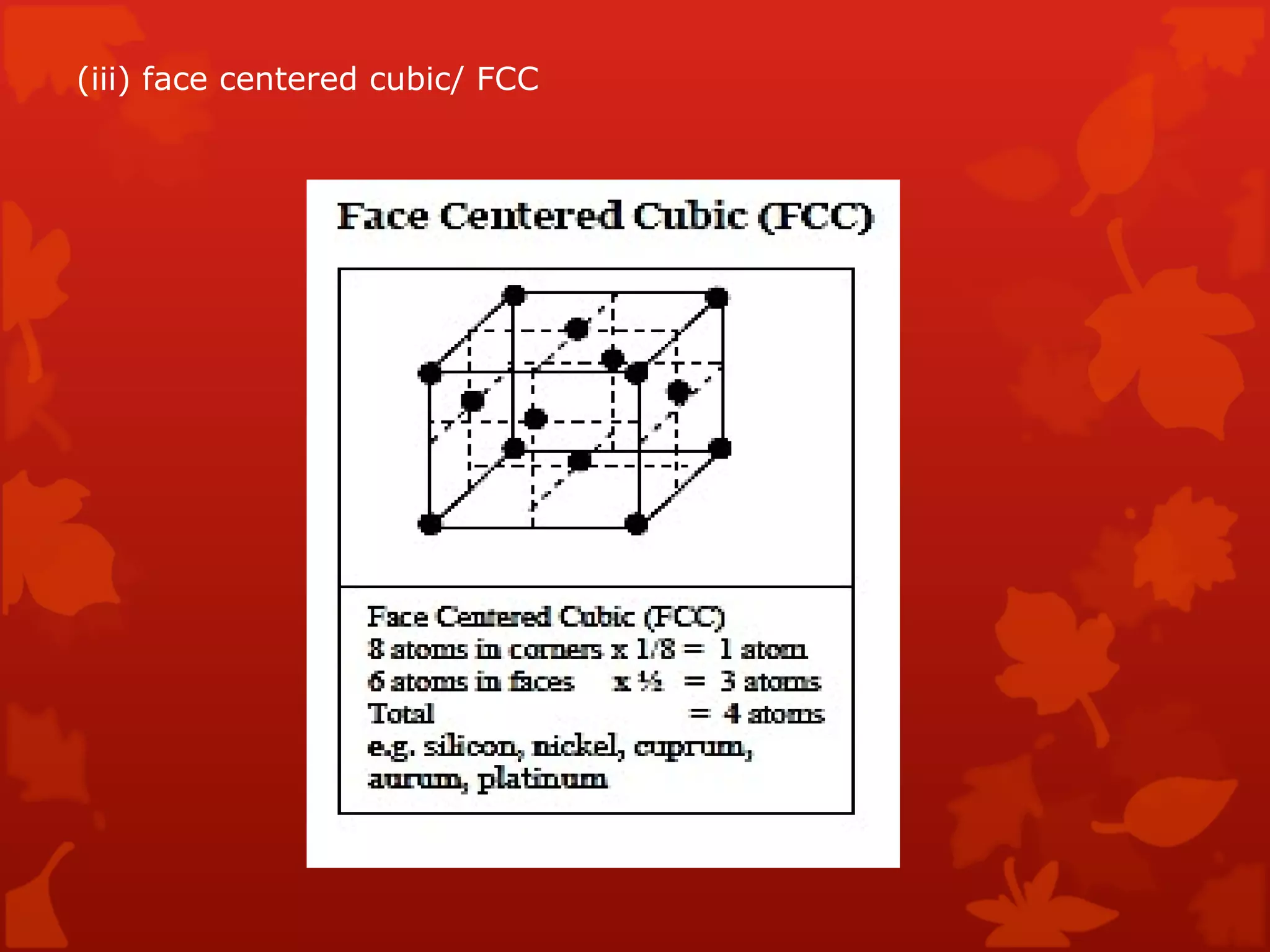
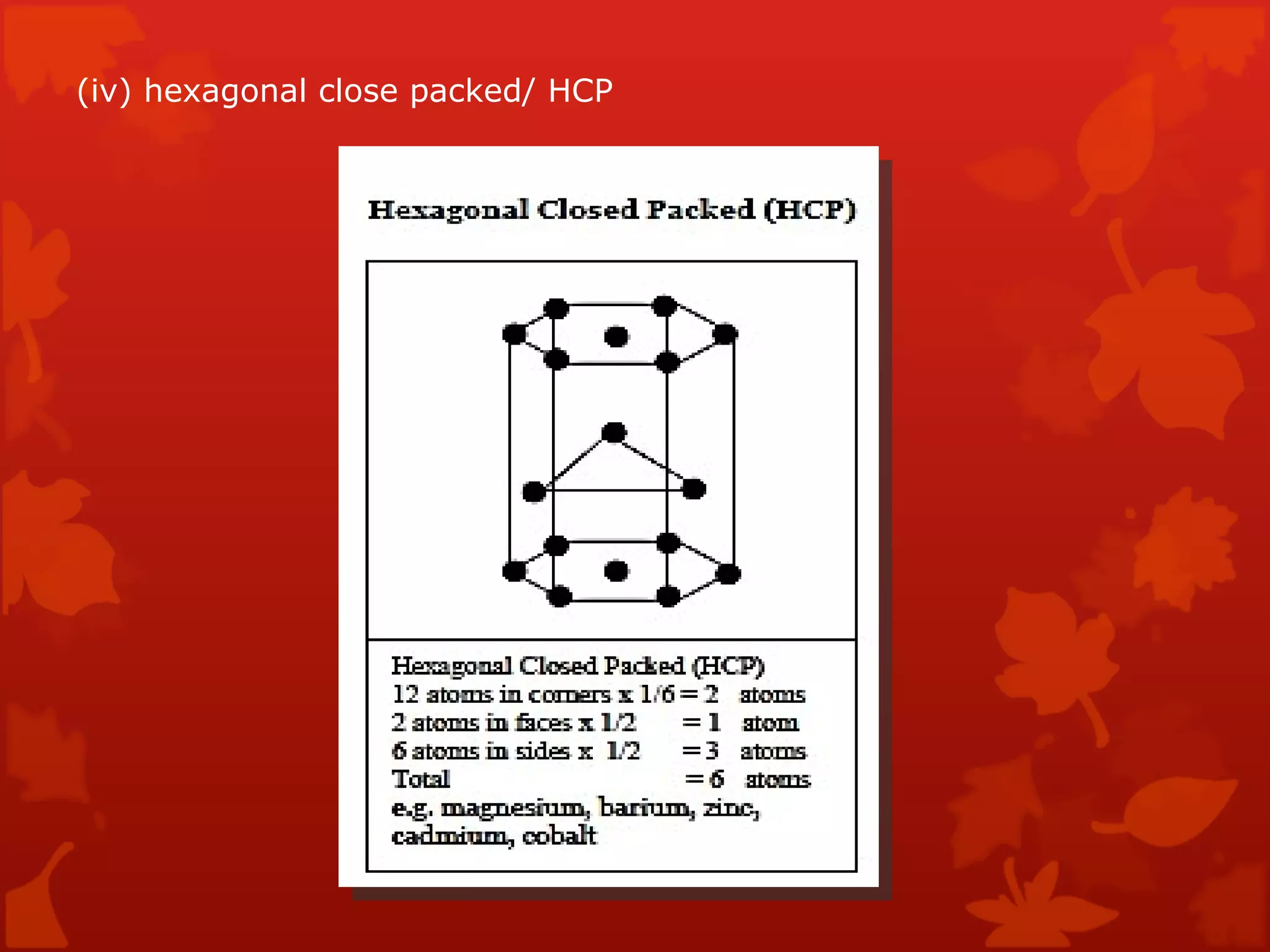
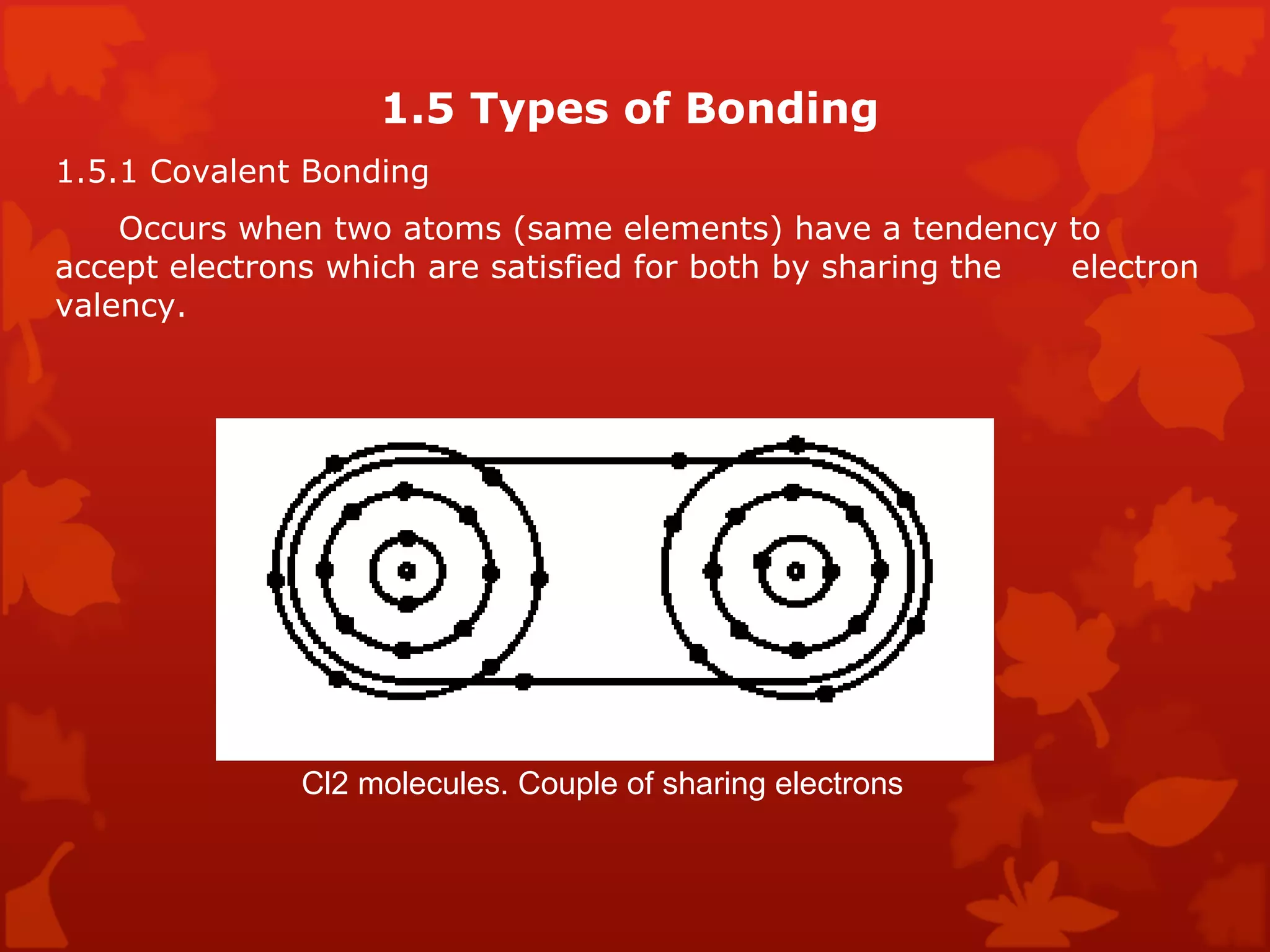
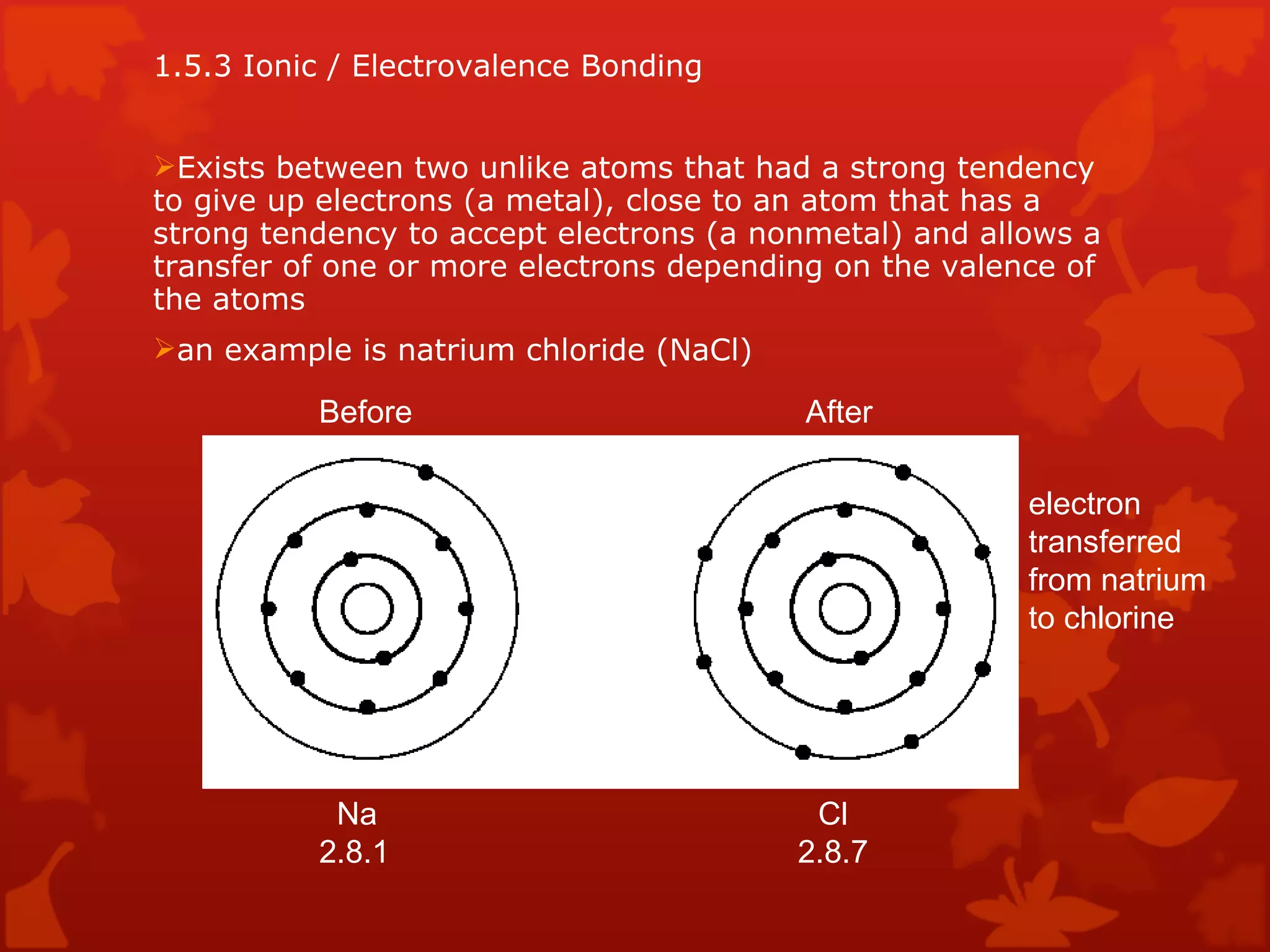
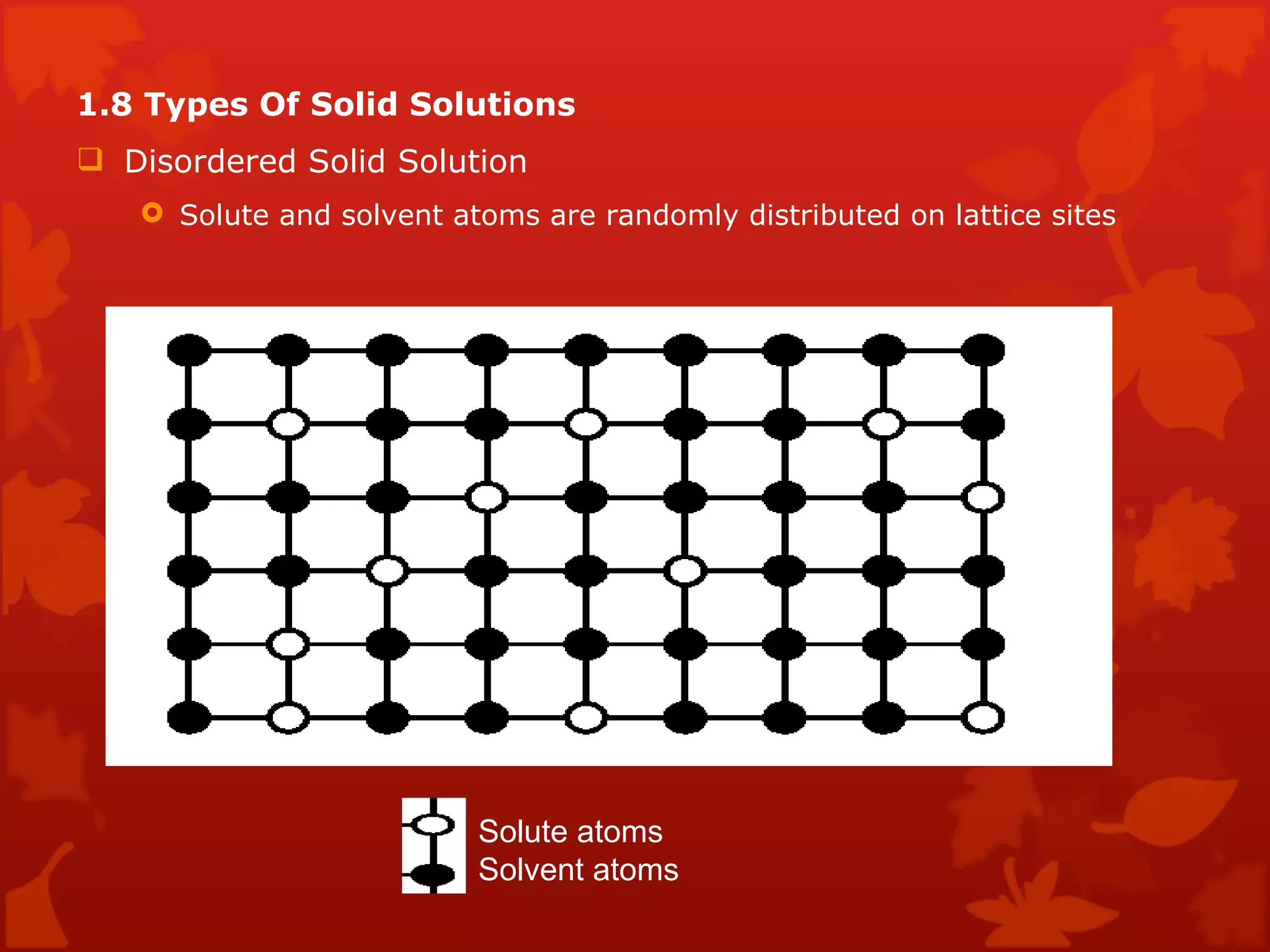
3. Different types of crystal structures are defined including body centered cubic, face centered cubic, and hexagonal close packed. Bonding types such as covalent, metallic, and ionic are also introduced.
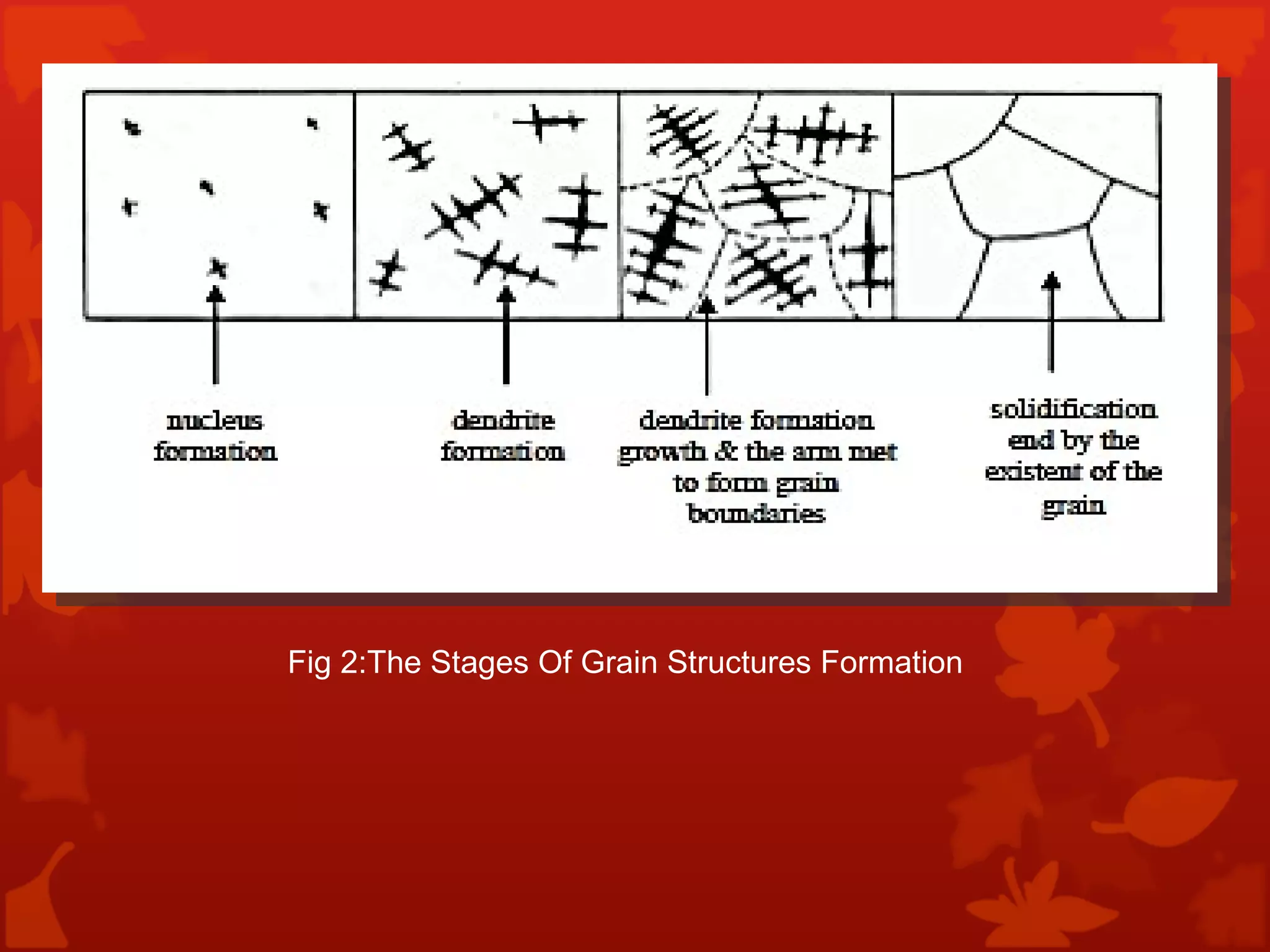
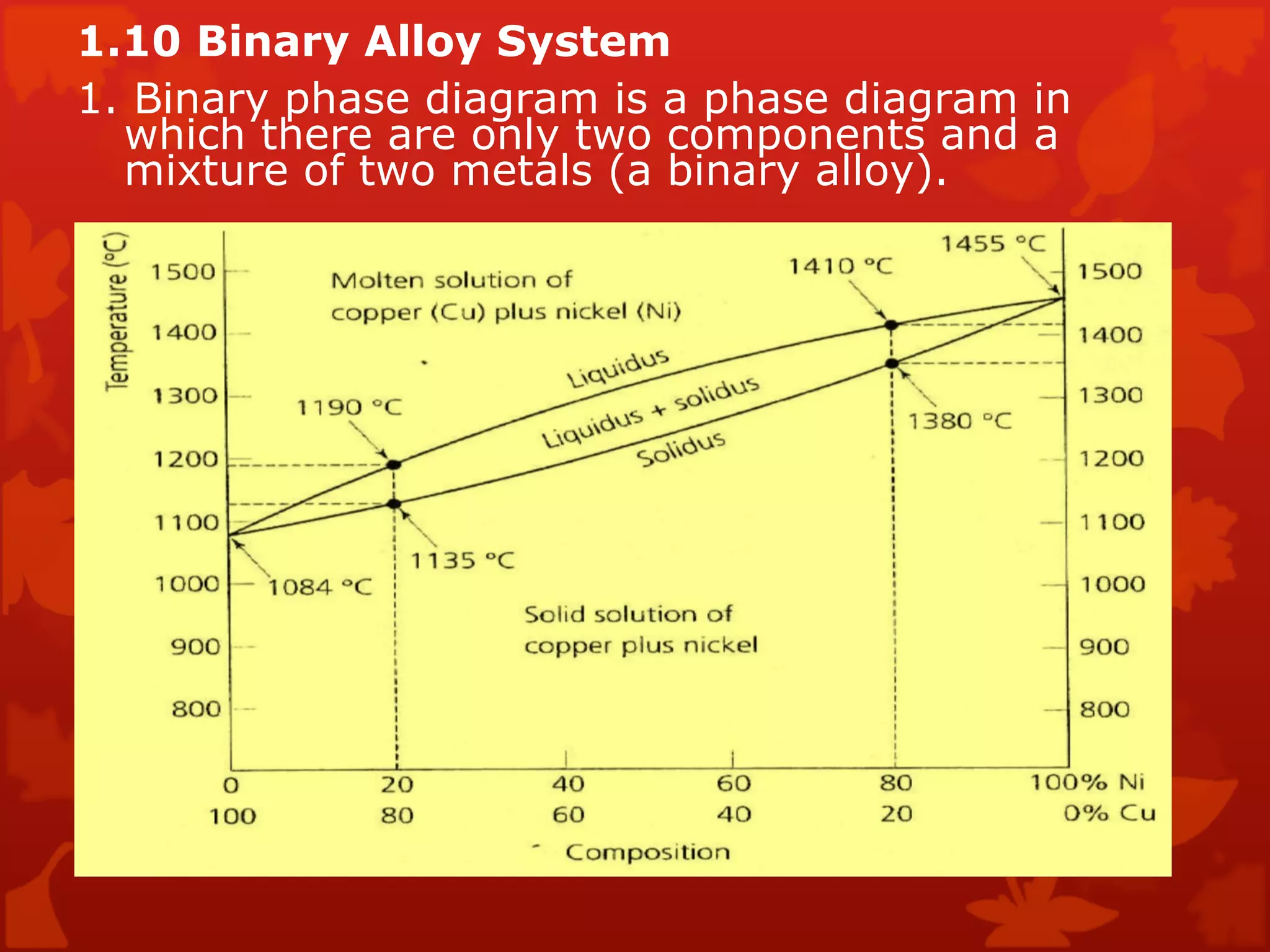
4. Terminology used in phase diagrams is defined including phases, equilibrium, composition, liquidus, and solidus. Binary alloy systems containing two components are also