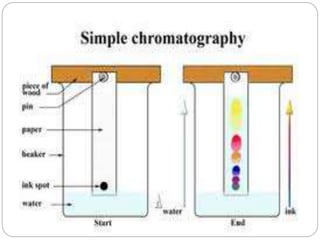
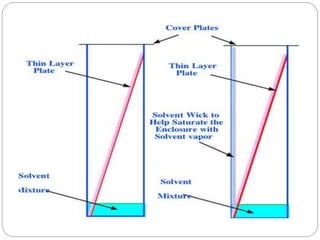
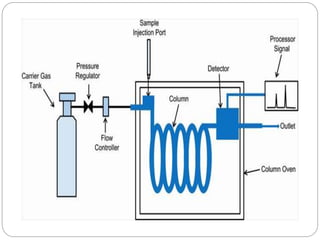
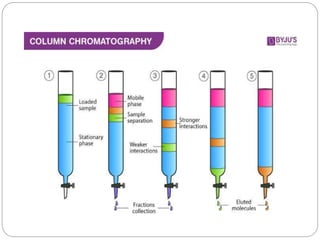
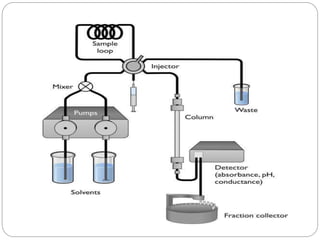
The document discusses different types of chromatography techniques used to separate mixtures. It describes chromatography as a collective term for laboratory methods that separate mixtures by dissolving them in a mobile phase that carries the mixture through a stationary phase. The key types discussed are paper chromatography, thin layer chromatography, gas chromatography, column chromatography, and high performance liquid chromatography. HPLC uses very small column packing and high pressure to improve separation speed over other chromatography methods.