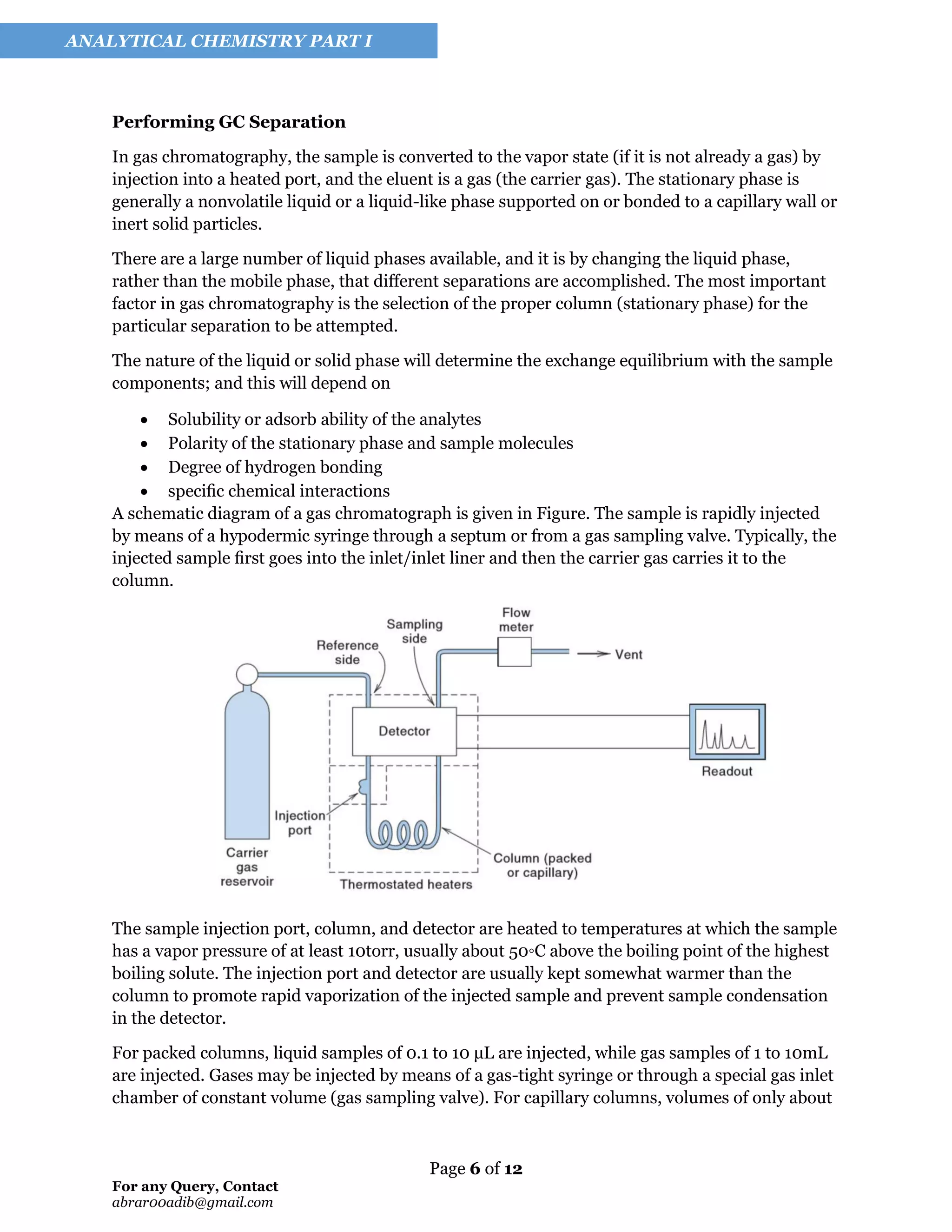
The document provides an overview of chromatography, defining it as a method of separation between stationary and mobile phases, which includes techniques such as liquid chromatography (LC) and gas chromatography (GC). It outlines the principles behind chromatographic separation, types of chromatography, and significant applications in fields like pharmaceuticals, forensic science, and environmental monitoring. Historical developments, including landmark contributions to the field, are also highlighted.

![Page 2 of 12
For any Query, Contact
abrar00adib@gmail.com
ANALYTICAL CHEMISTRY PART I
Principle of Chromatographic Separation
While the mechanisms of retention for various types of chromatography differ, they are all based
on the dynamic distribution of an analyte between a fixed stationary phase and a flowing mobile
phase. Each analyte will have a certain affinity for each phase.
Figure illustrates the separation of components in a mixture on a chromatographic column. A
small volume of sample is placed at the top of the column, which is filled with particles
constituting the stationary phase and the solvent. Rather than an equilibrium-based “plate view”
of chromatography, many hold that a “rate view” of chromatography to be more rigorous: in this
view, the partition ratio is simply the ratio of the time a solute spends in the stationary phase to
that it spends in the mobile phase.
More solvent, function in gas mobile phase, is added to the top of the column and percolates
through the column. The individual components interact with the stationary phase to different
degrees.
There is nominally an equilibrium between two phases, one mobile and one stationary. By
continually adding mobile phase, the analyte will distribute between the two phases and
eventually be eluted, and if the distribution is sufficiently different for the different substances,
they will be separated.
Here the equilibrium state is described as-
𝑥 𝑚 ⇌ 𝑥 𝑠
The distribution equilibrium is described by the distribution constant.
𝐾𝑒 =
[𝑥] 𝑠
[𝑥] 𝑚](https://image.slidesharecdn.com/chromatography-181005183456/75/Chromatography-2-2048.jpg)
![Page 3 of 12
For any Query, Contact
abrar00adib@gmail.com
ANALYTICAL CHEMISTRY PART I
Where, [x]s is the concentration of component x in stationary phase at equilibrium
[x]m is the concentration in the mobile phase.
Figure illustrates the distribution of two species A and B along a column as they move down the
column
The distribution of the analyte between the two phases is governed by many factors:
• Temperature
• Type of compound
• Stationary phases
• Mobile phases
Thin Layer Chromatography (TLC)
Thin-layer chromatography (TLC) is a planar form of chromatography widely used for rapid
qualitative analysis. It can also be used in a high-performance mode (HPTLC). Quantitative
analysis is also possible, although the technique is most widely used for rapid screening.](https://image.slidesharecdn.com/chromatography-181005183456/75/Chromatography-3-2048.jpg)