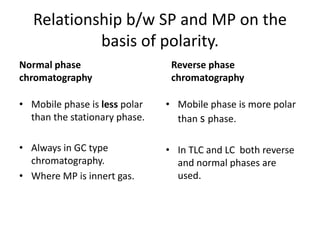
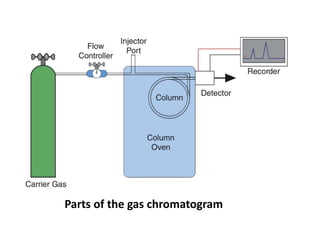
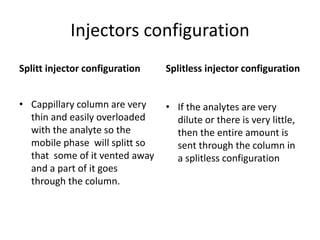
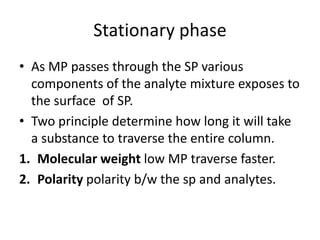
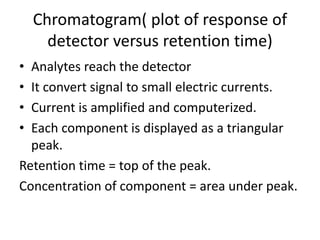
Chromatography is a technique primarily used for separating mixtures and components at micro or nano levels, developed by Russian botanist Michael Tswett in 1904. It operates on the principle of differing affinities of analyte components for stationary and mobile phases, allowing for sensitive quantitative analysis. Modern chromatography utilizes detectors for non-colored analytes, and parameters like temperature, polarity, and molecular weight affect retention times during separation.