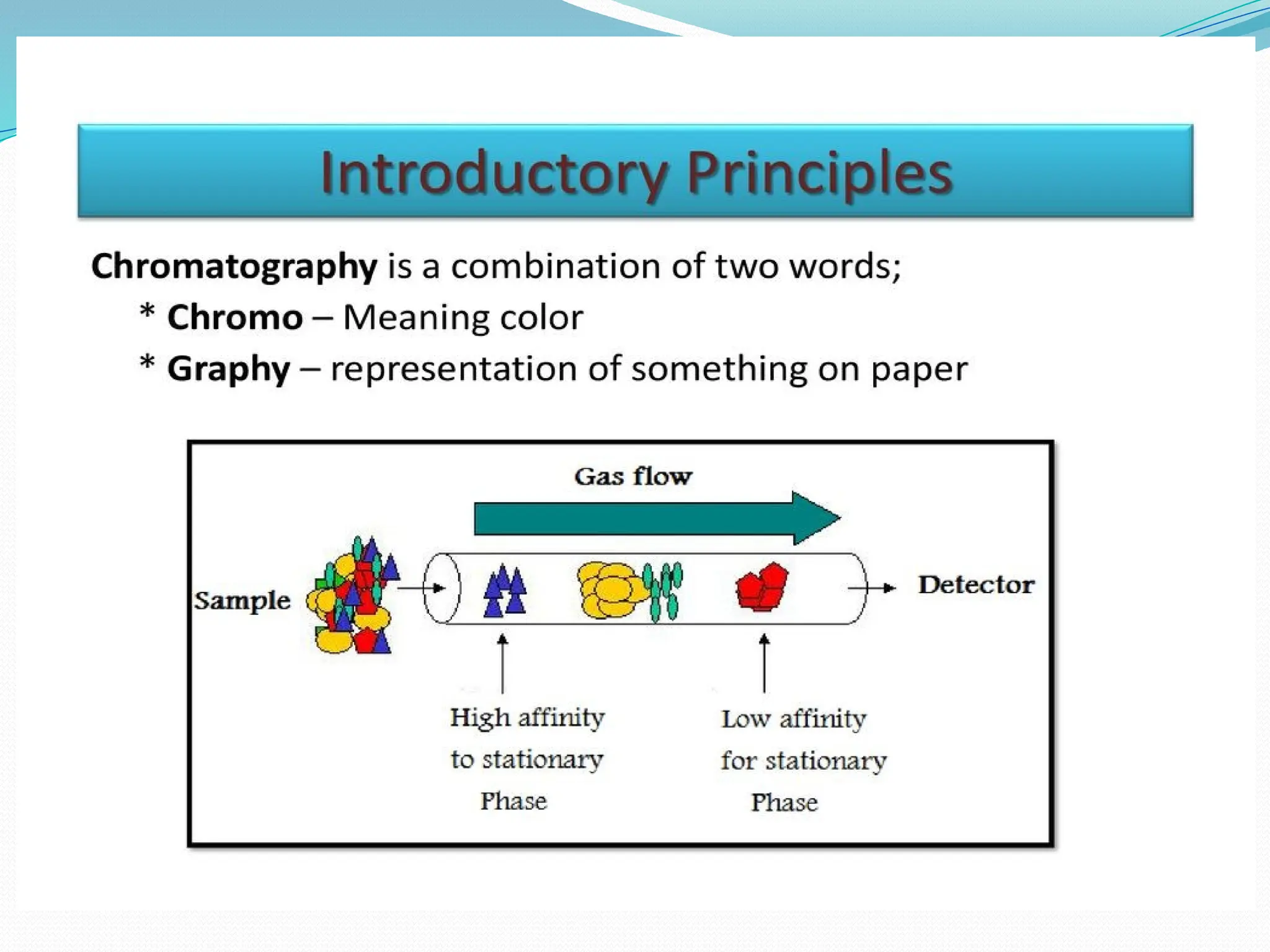
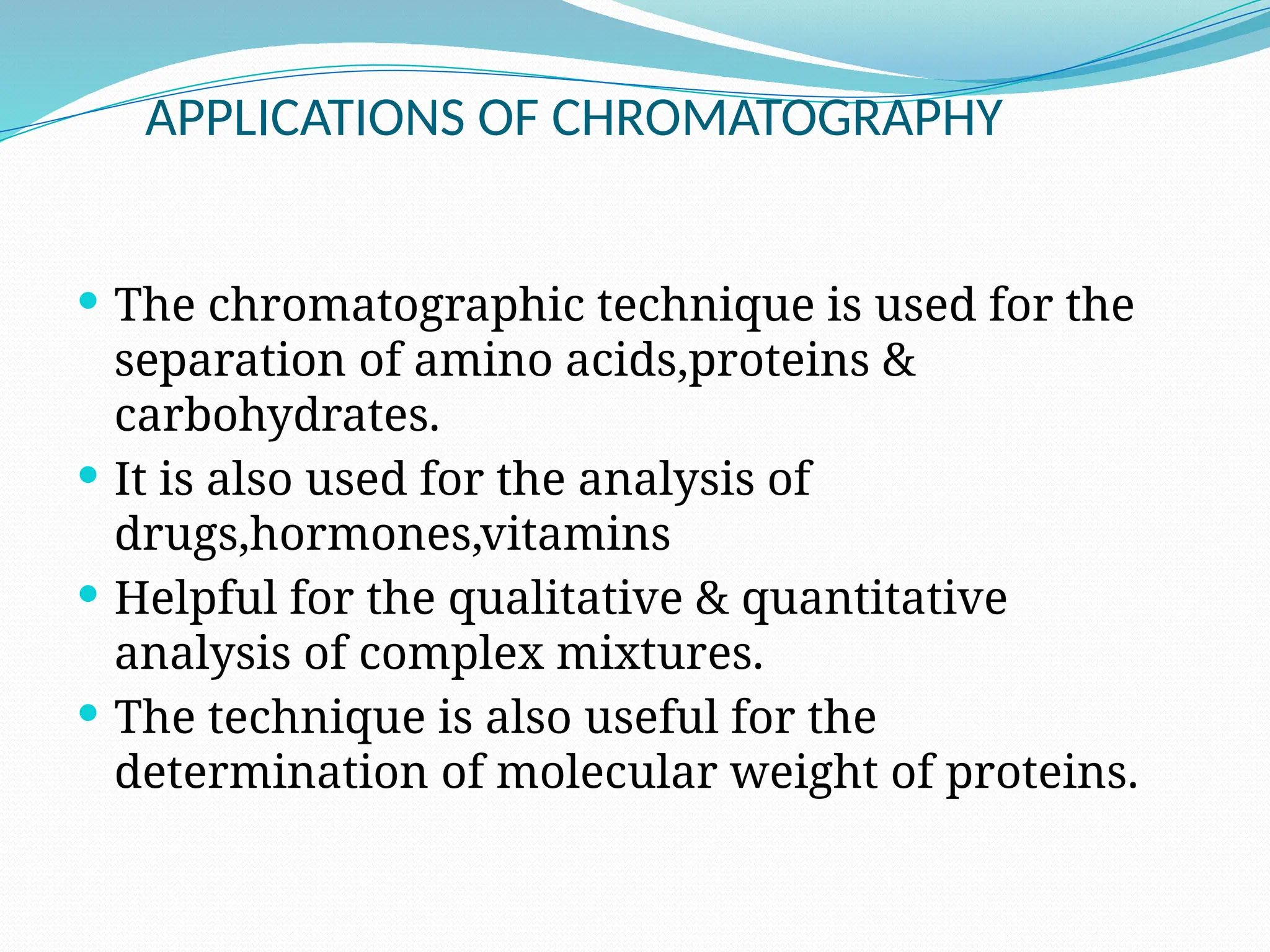
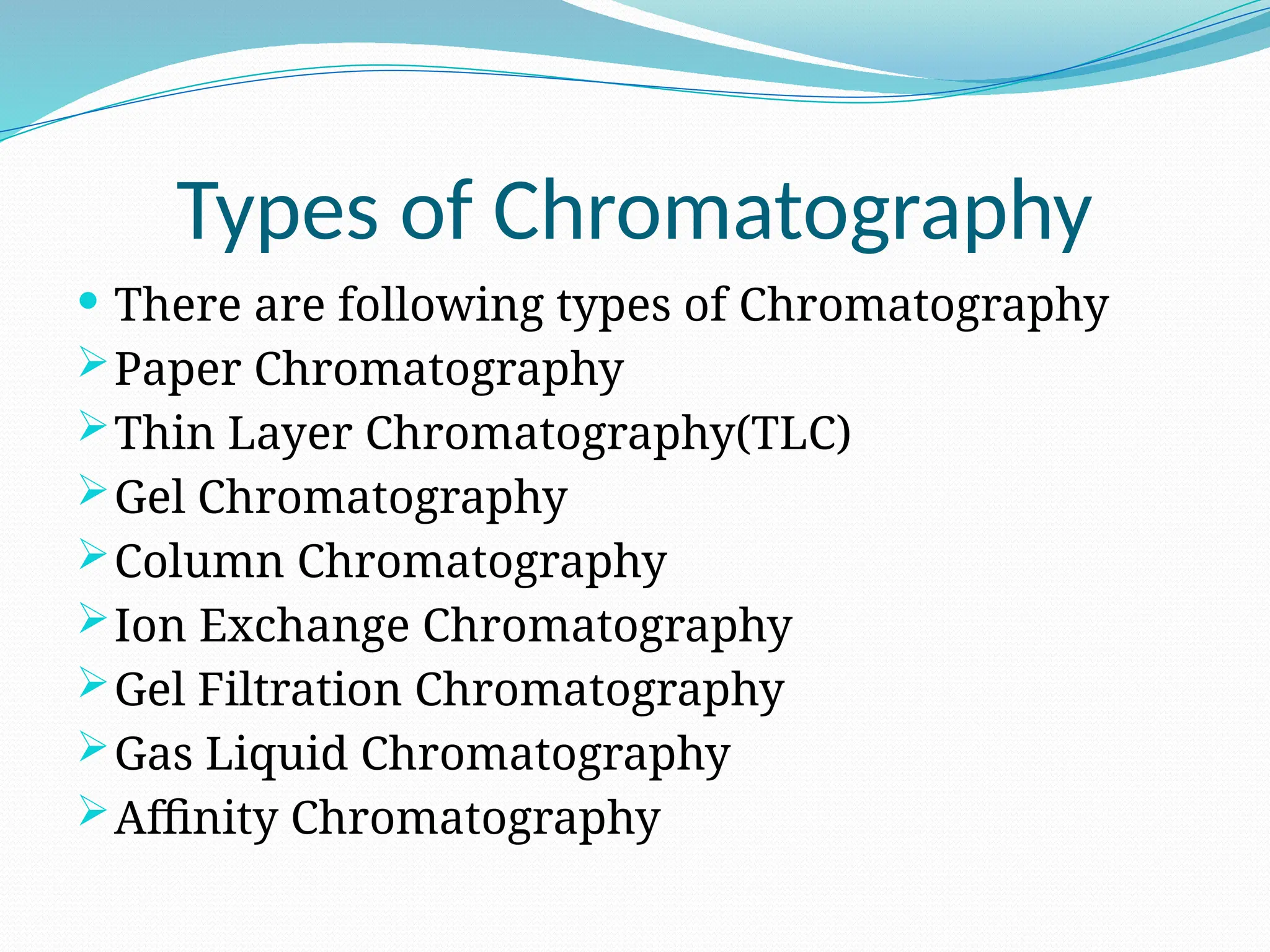
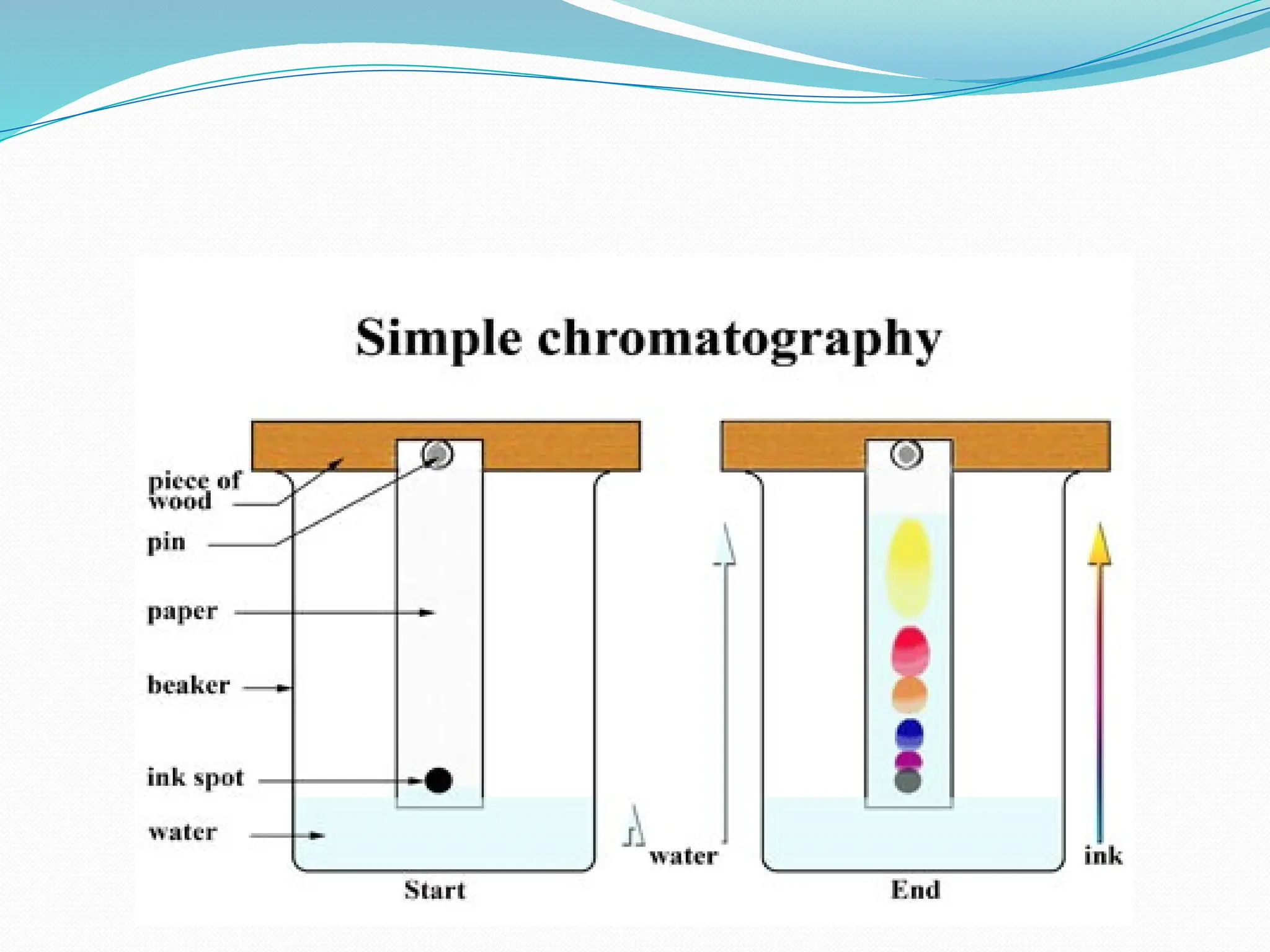
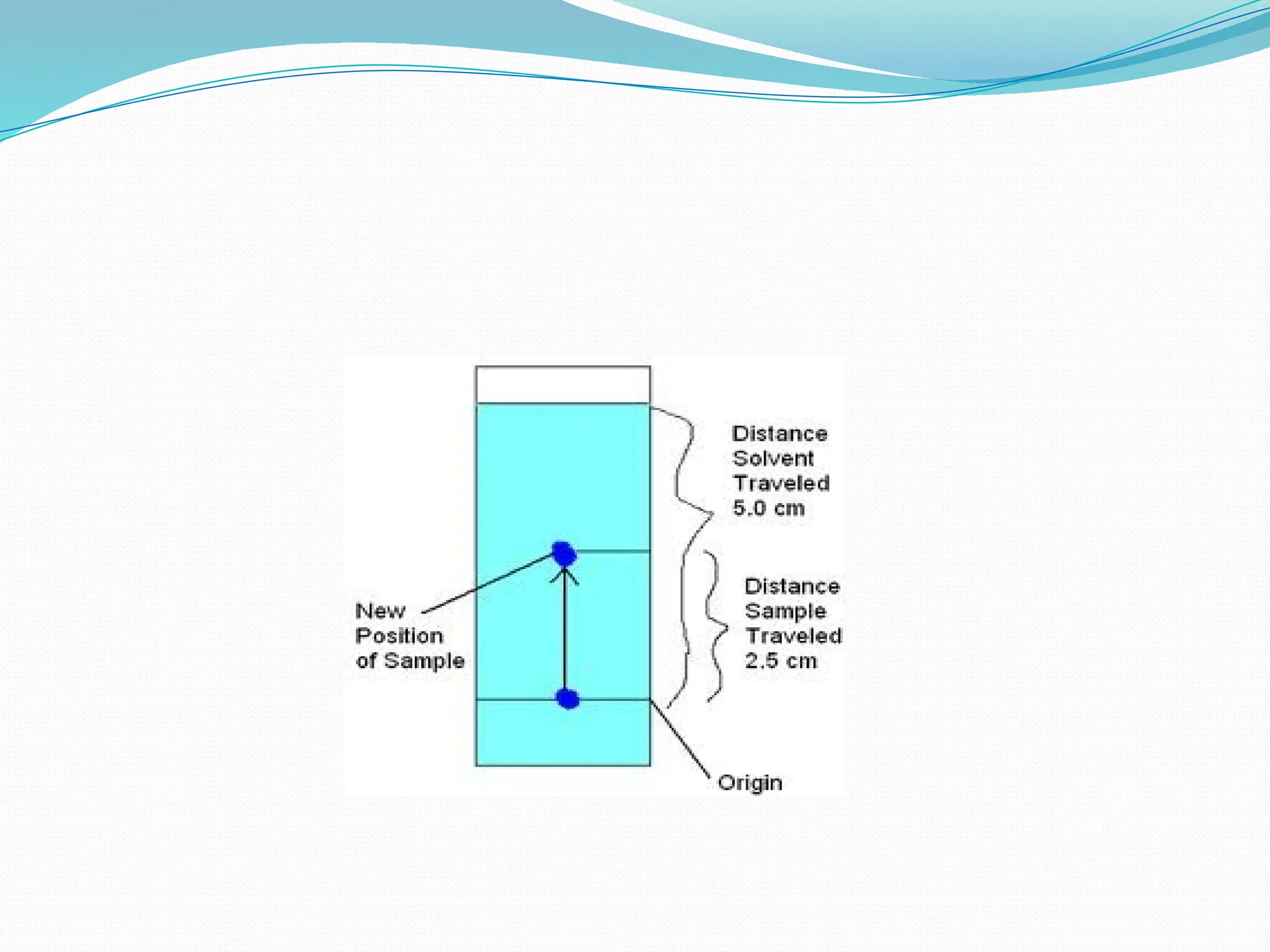
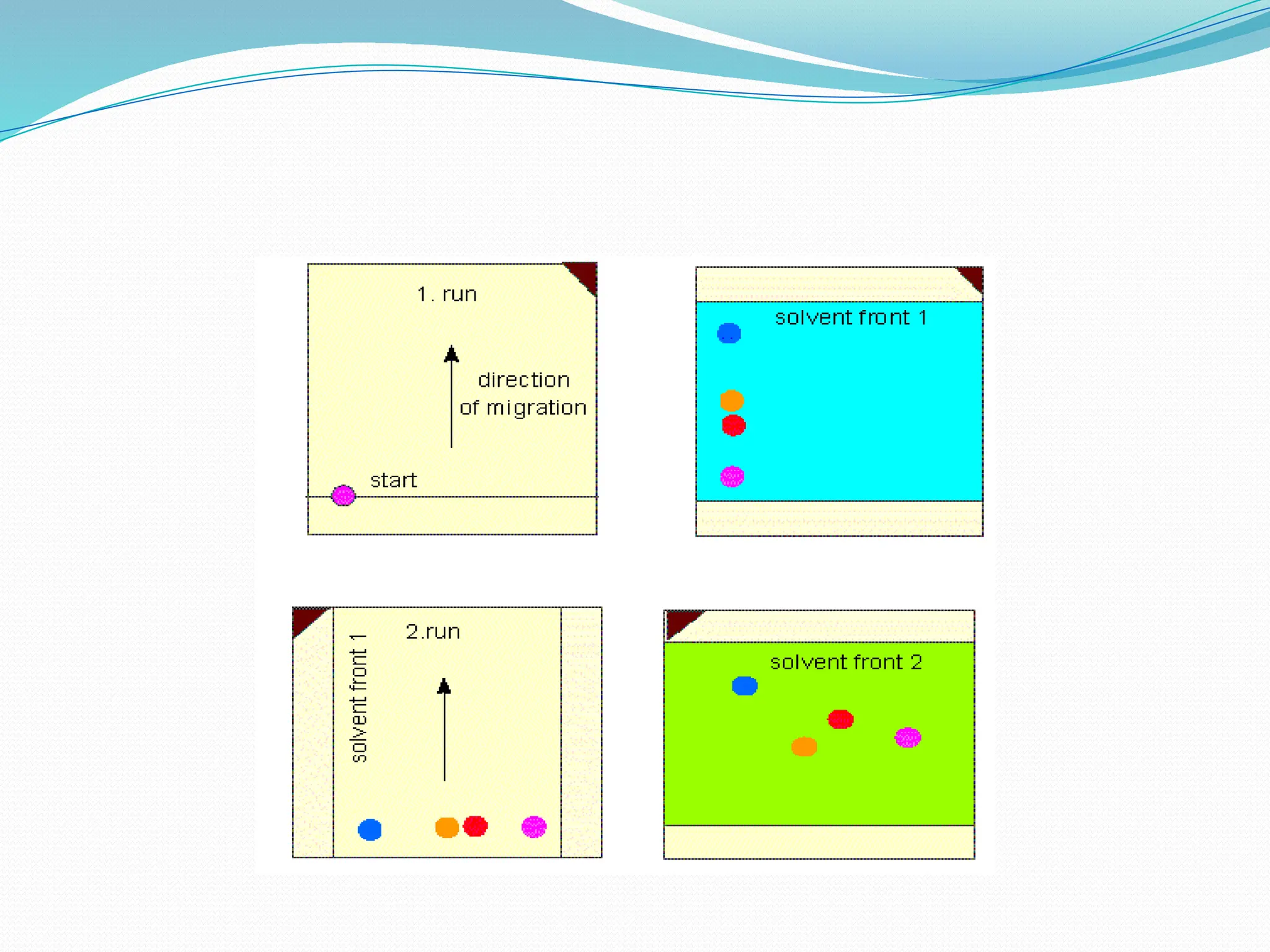
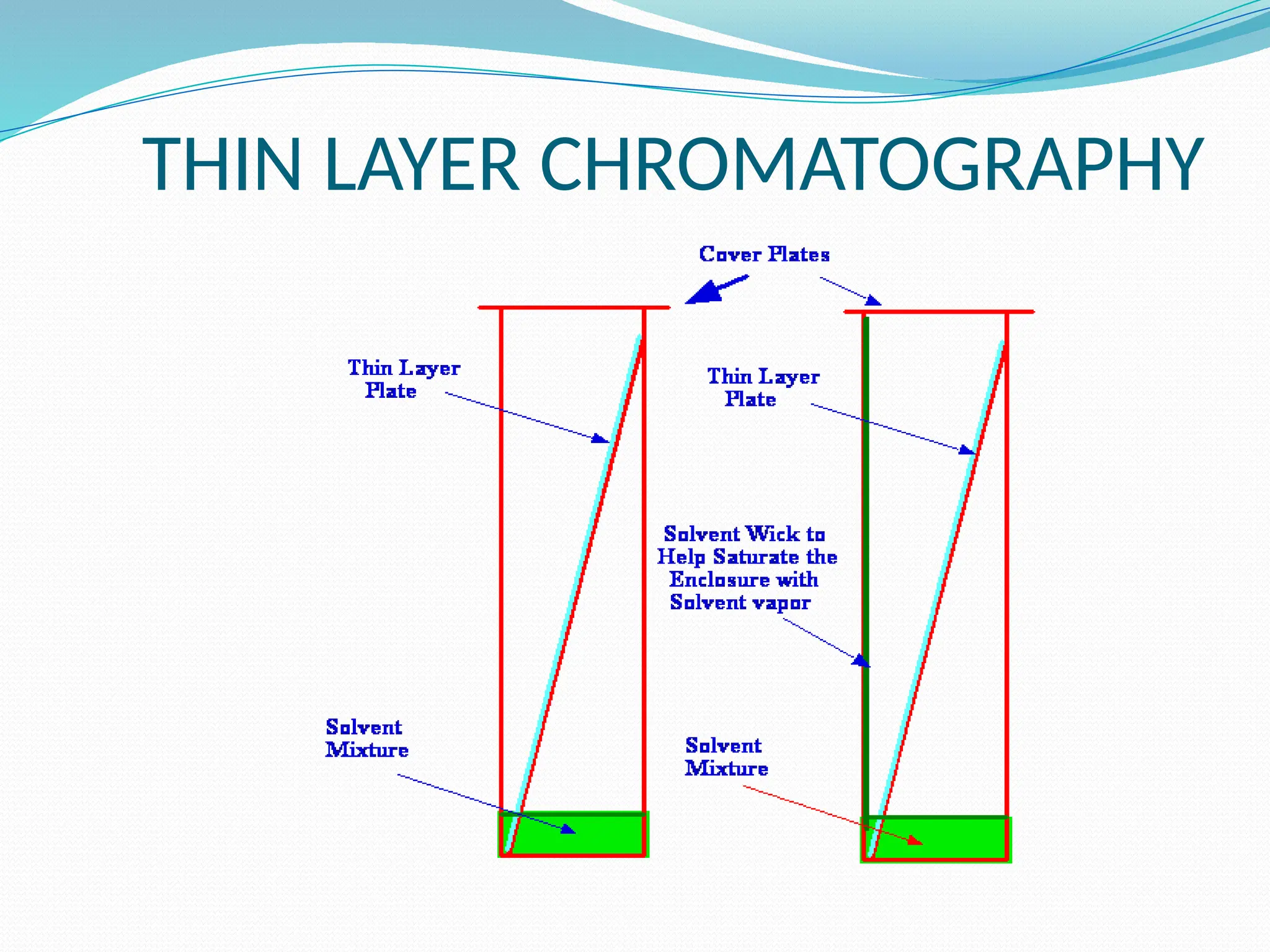
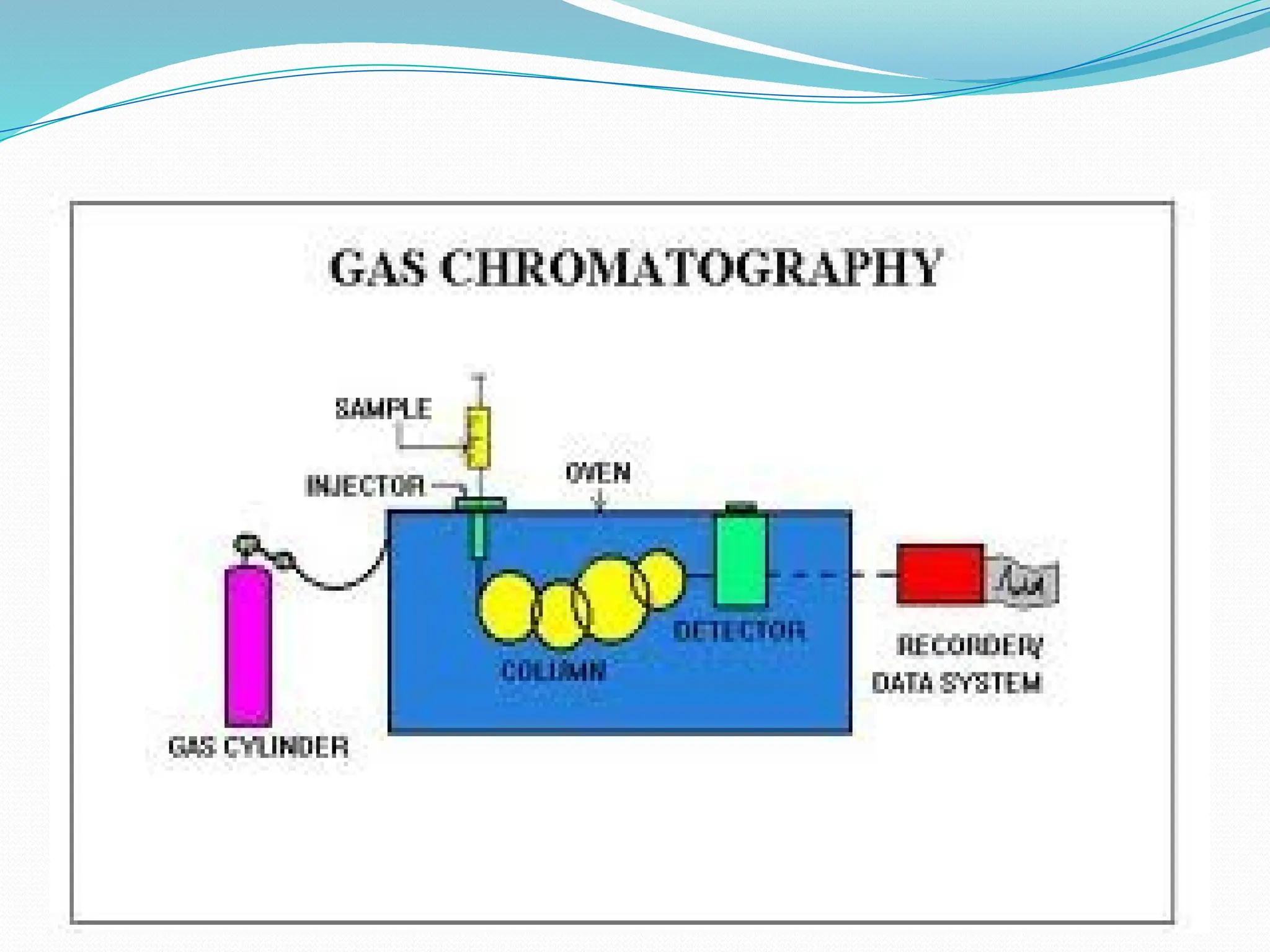
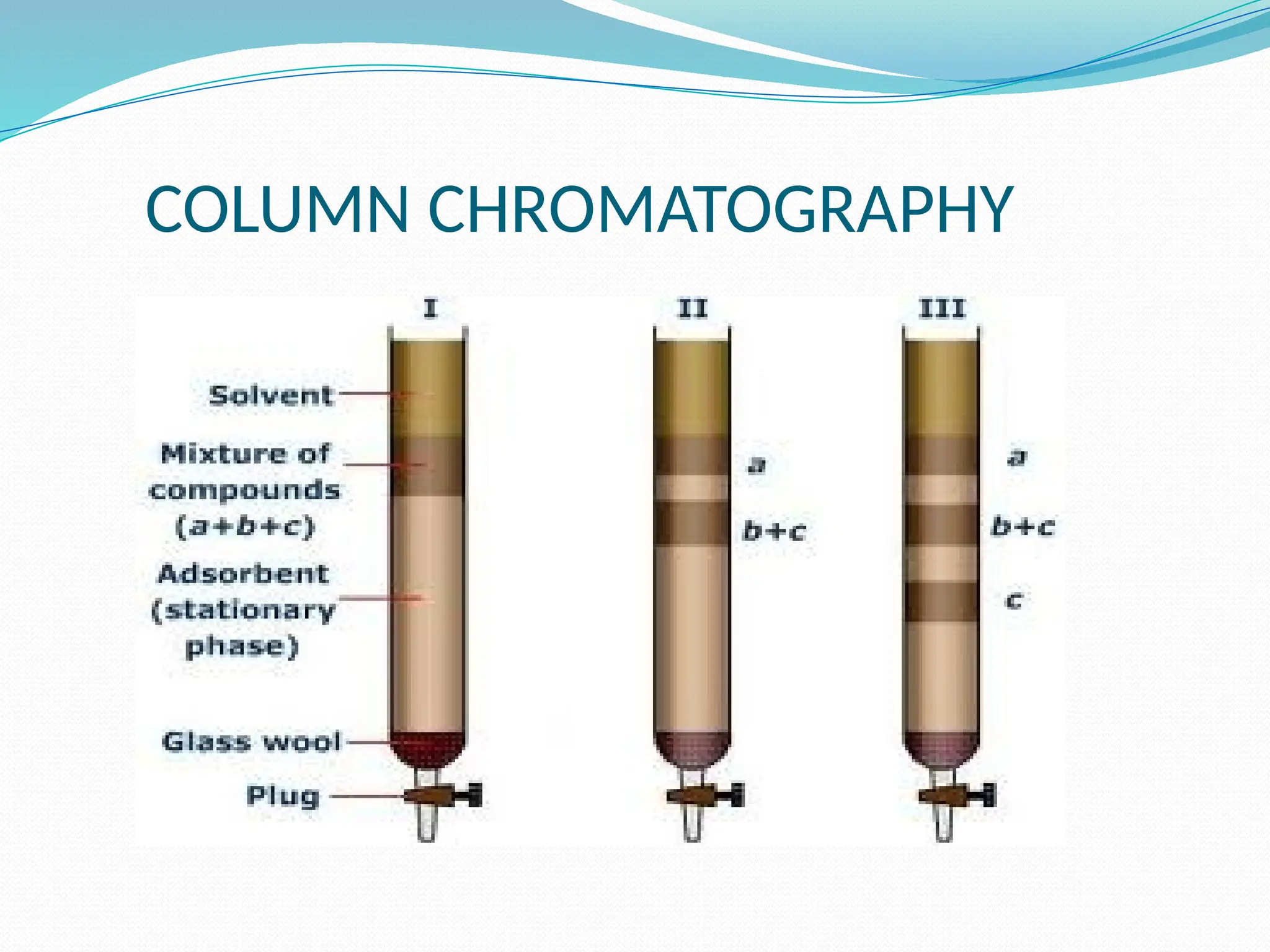
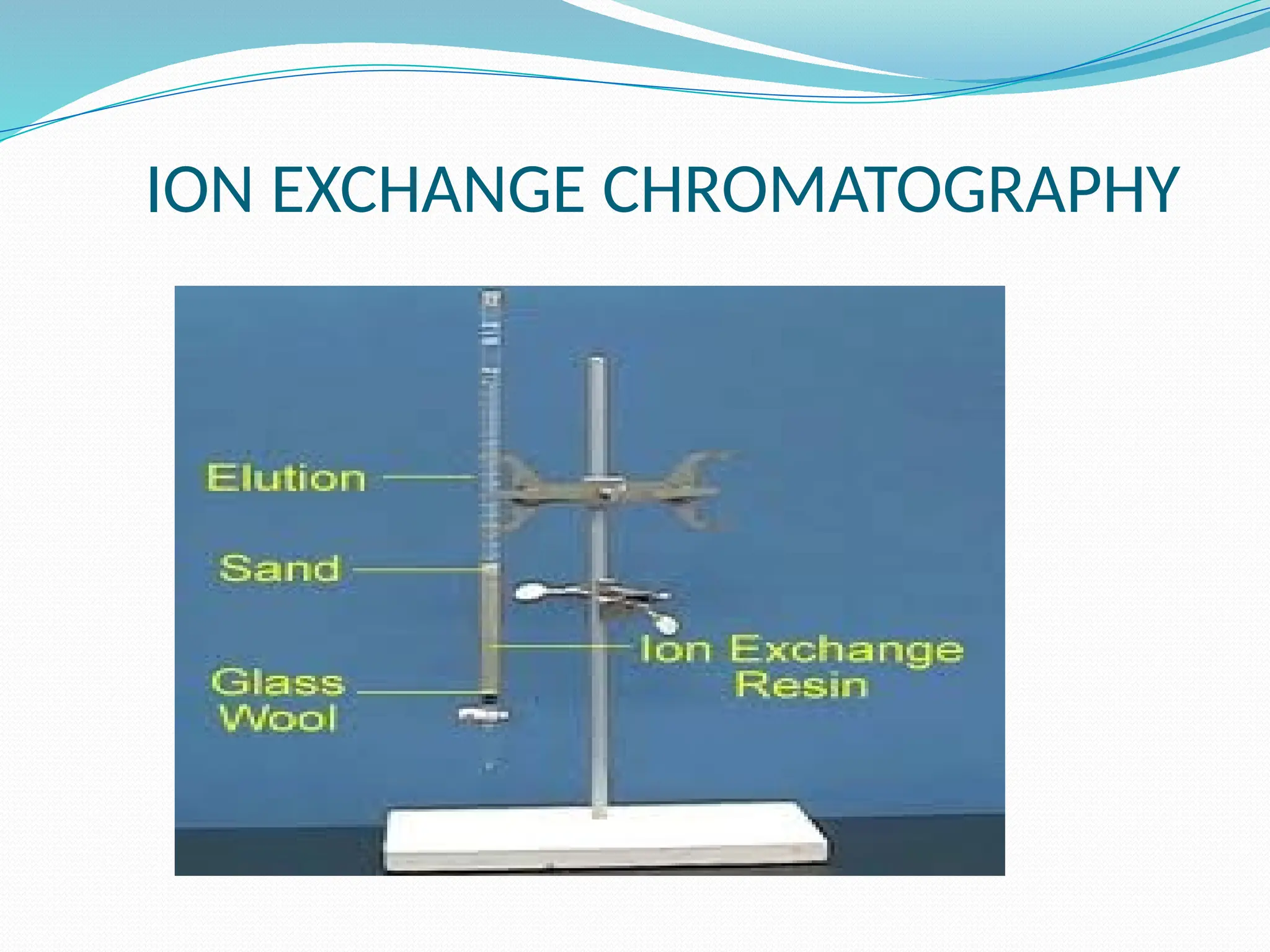
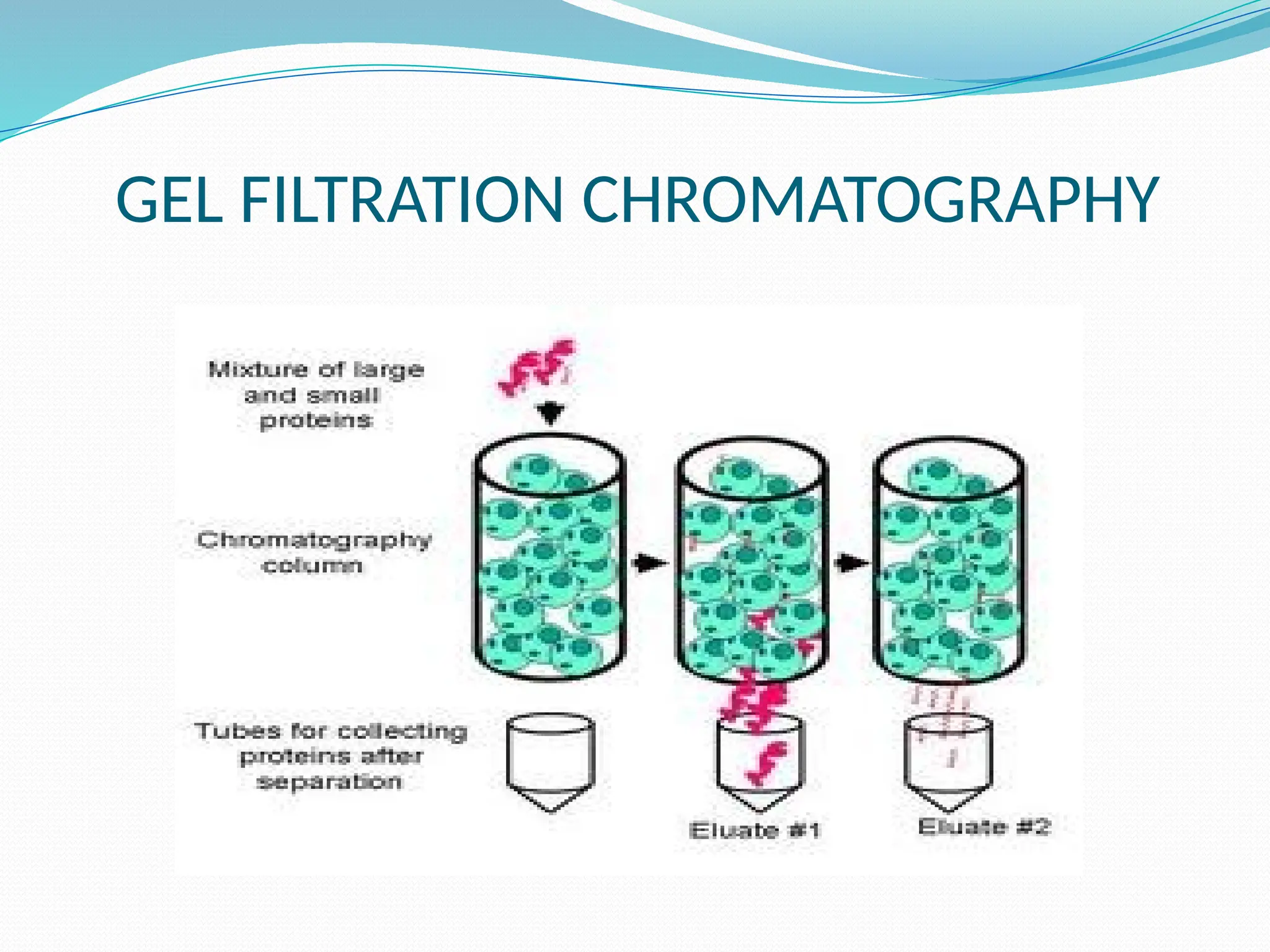
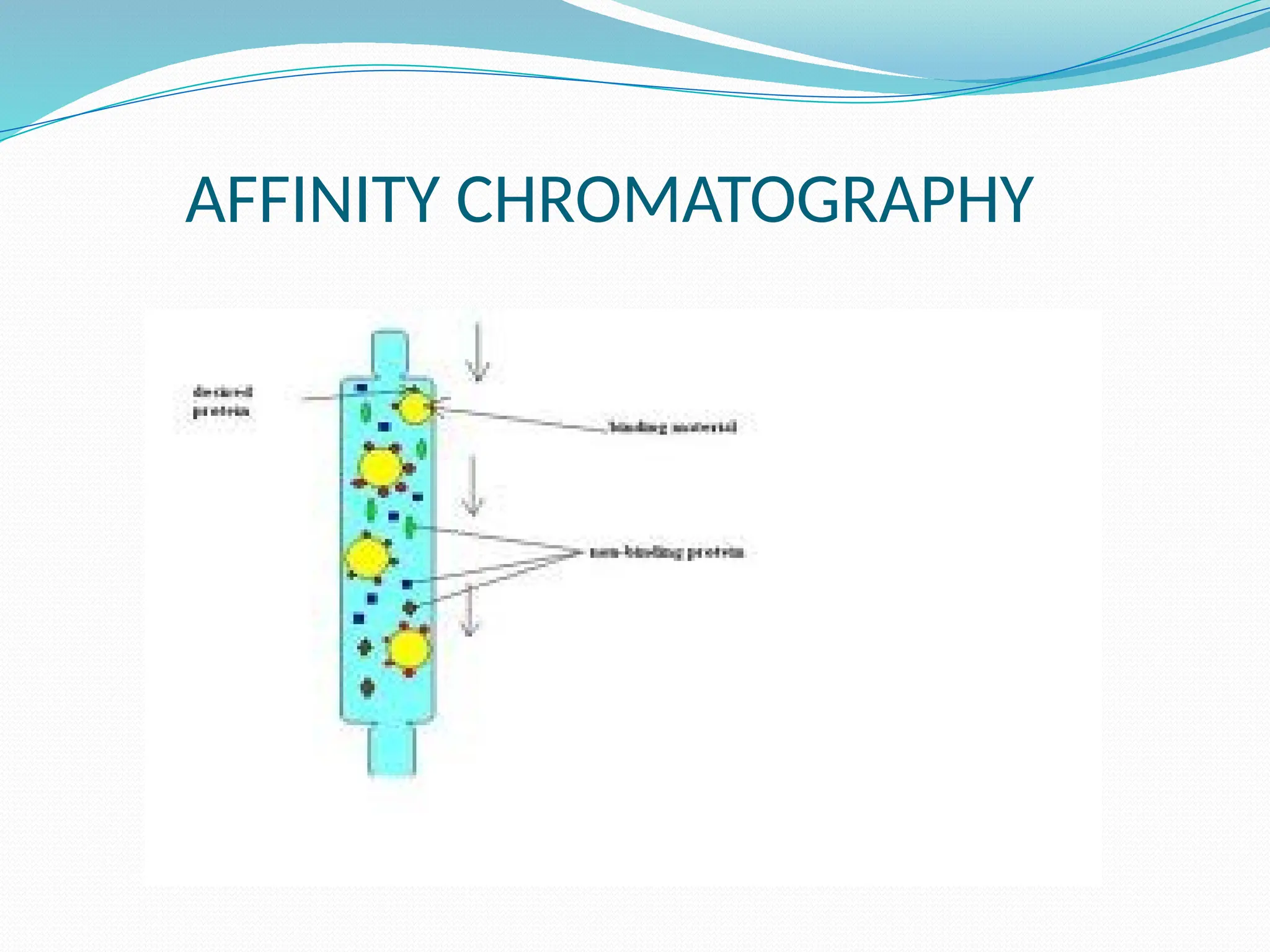
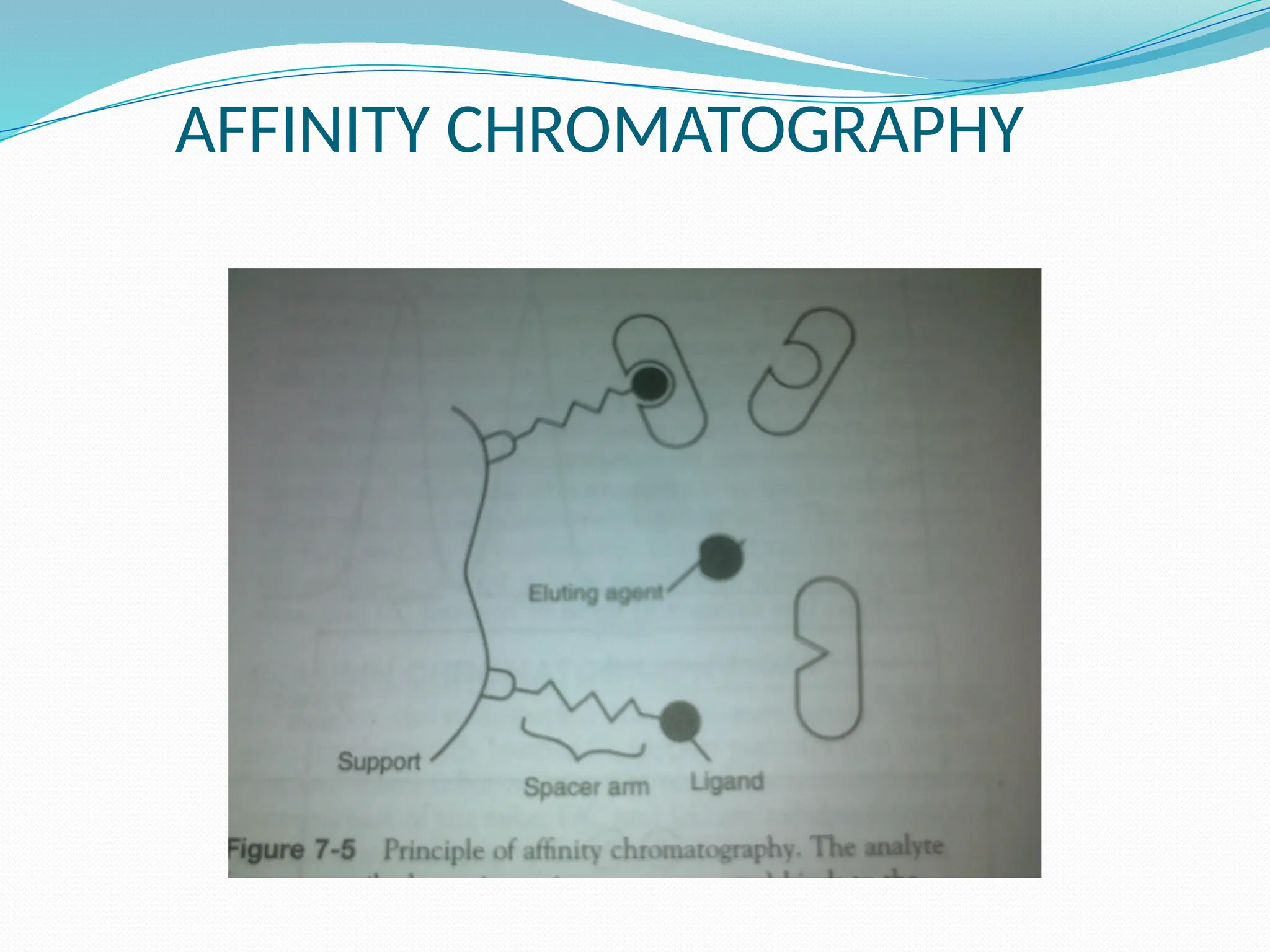
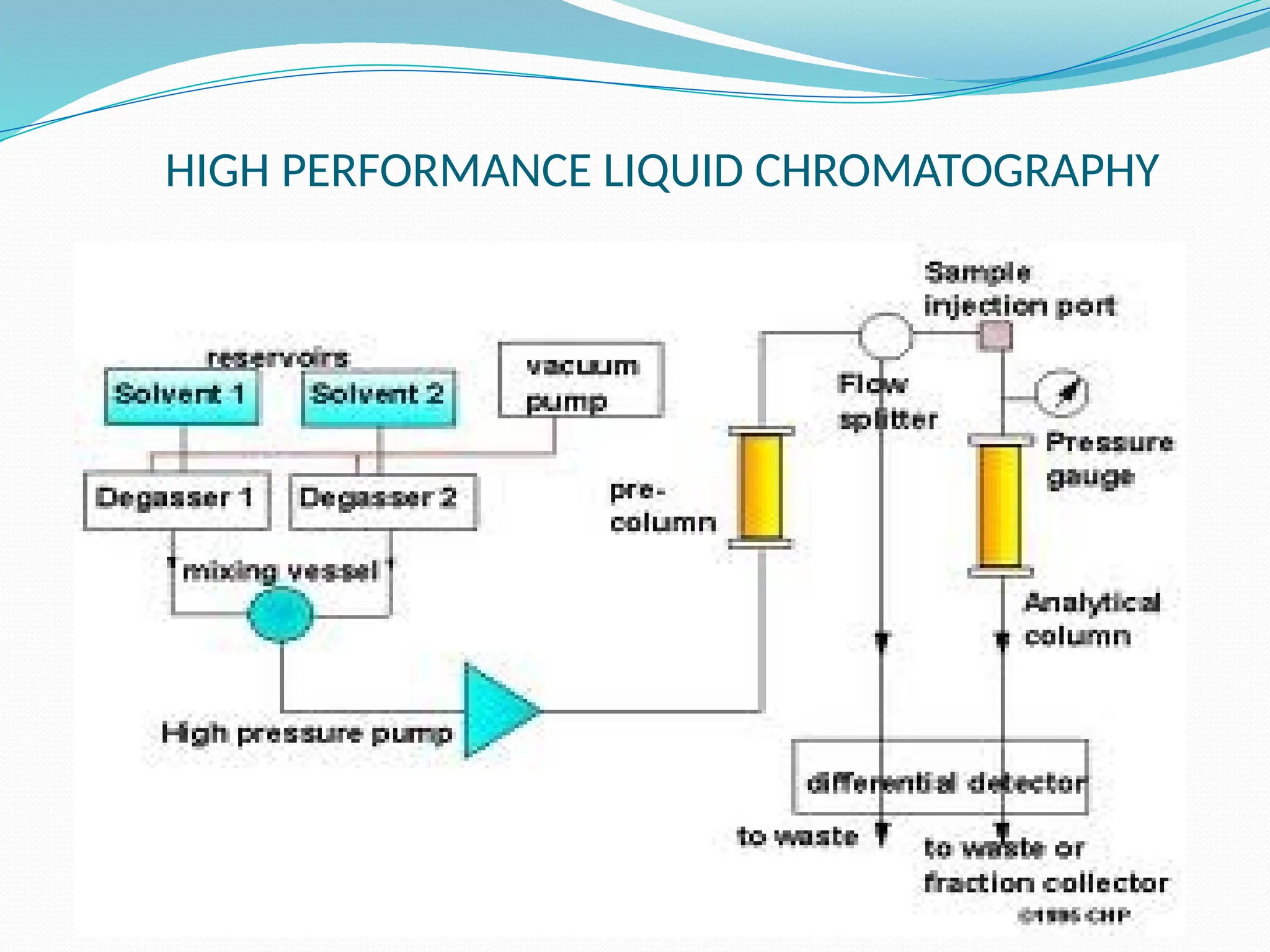
Chromatography is a laboratory technique used for separating mixtures based on differential partitioning between a mobile phase and a stationary phase, first introduced by Mikhail Tsvet in 1900. There are various types, including paper, thin-layer, gas-liquid, column, ion exchange, gel filtration, affinity chromatography, and high-performance liquid chromatography, each suited for different applications like separating amino acids, proteins, and analyzing drugs or vitamins. The technique involves specific interactions that allow for the analysis and purification of complex mixtures in various scientific fields.