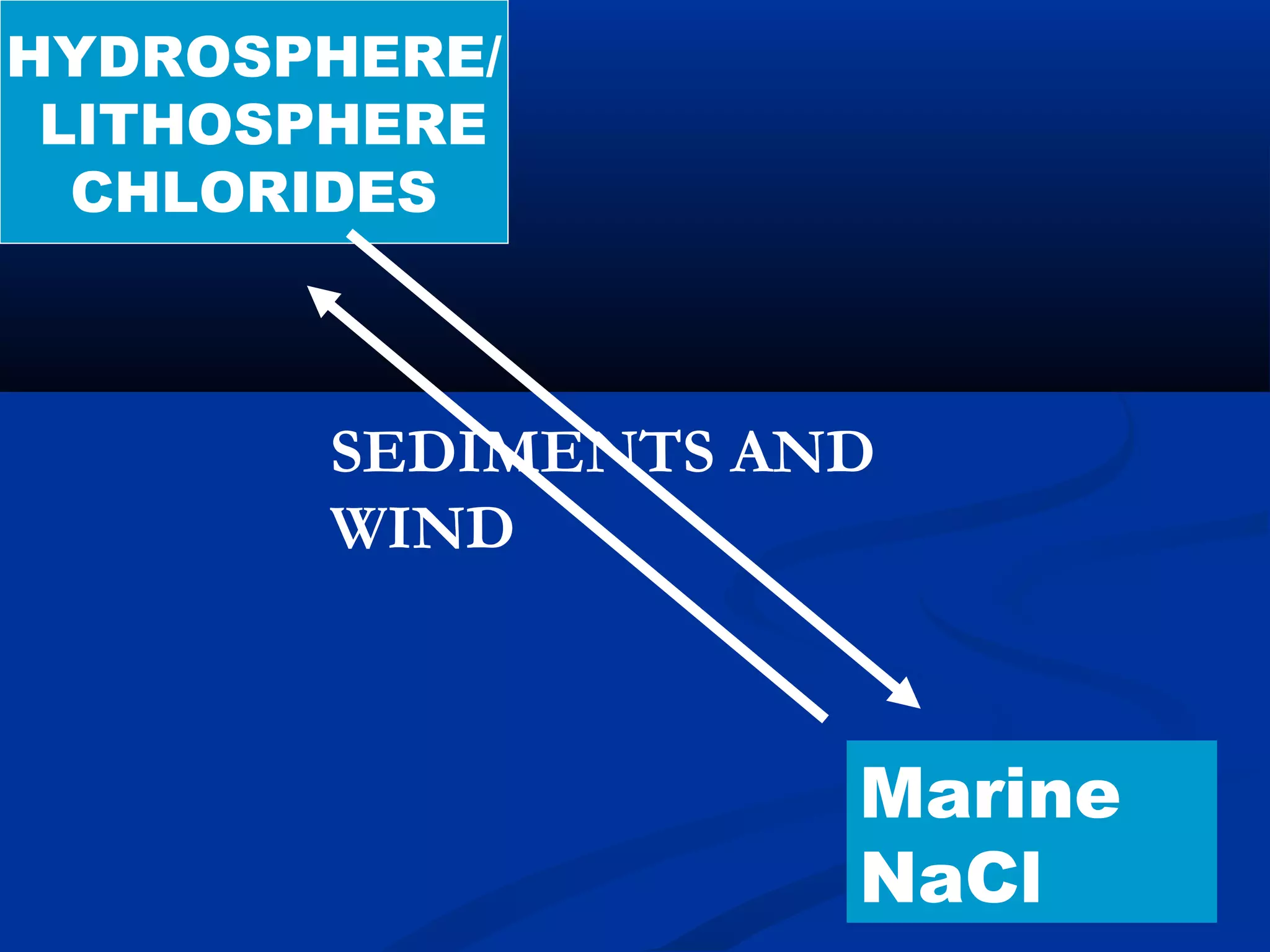
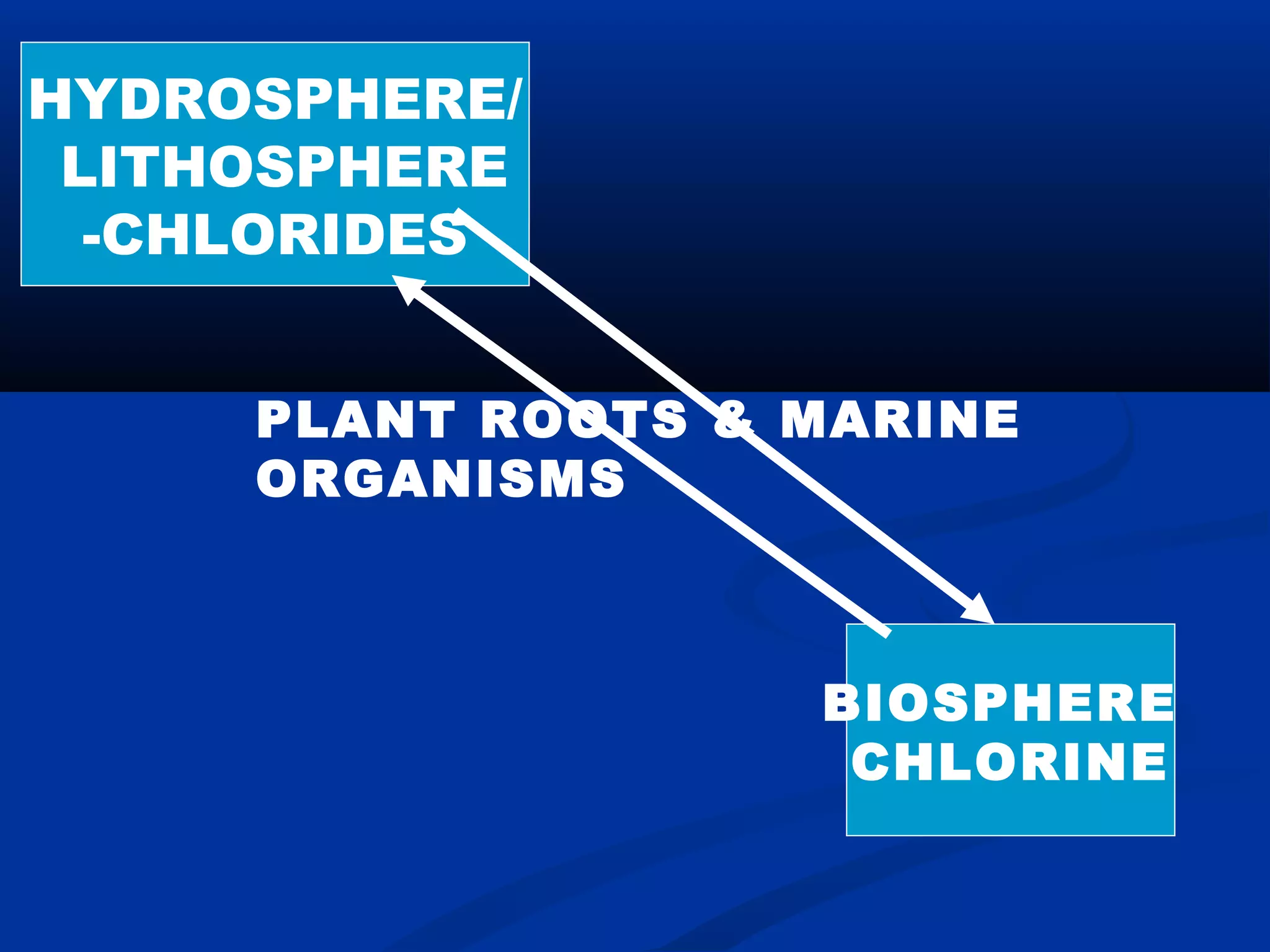
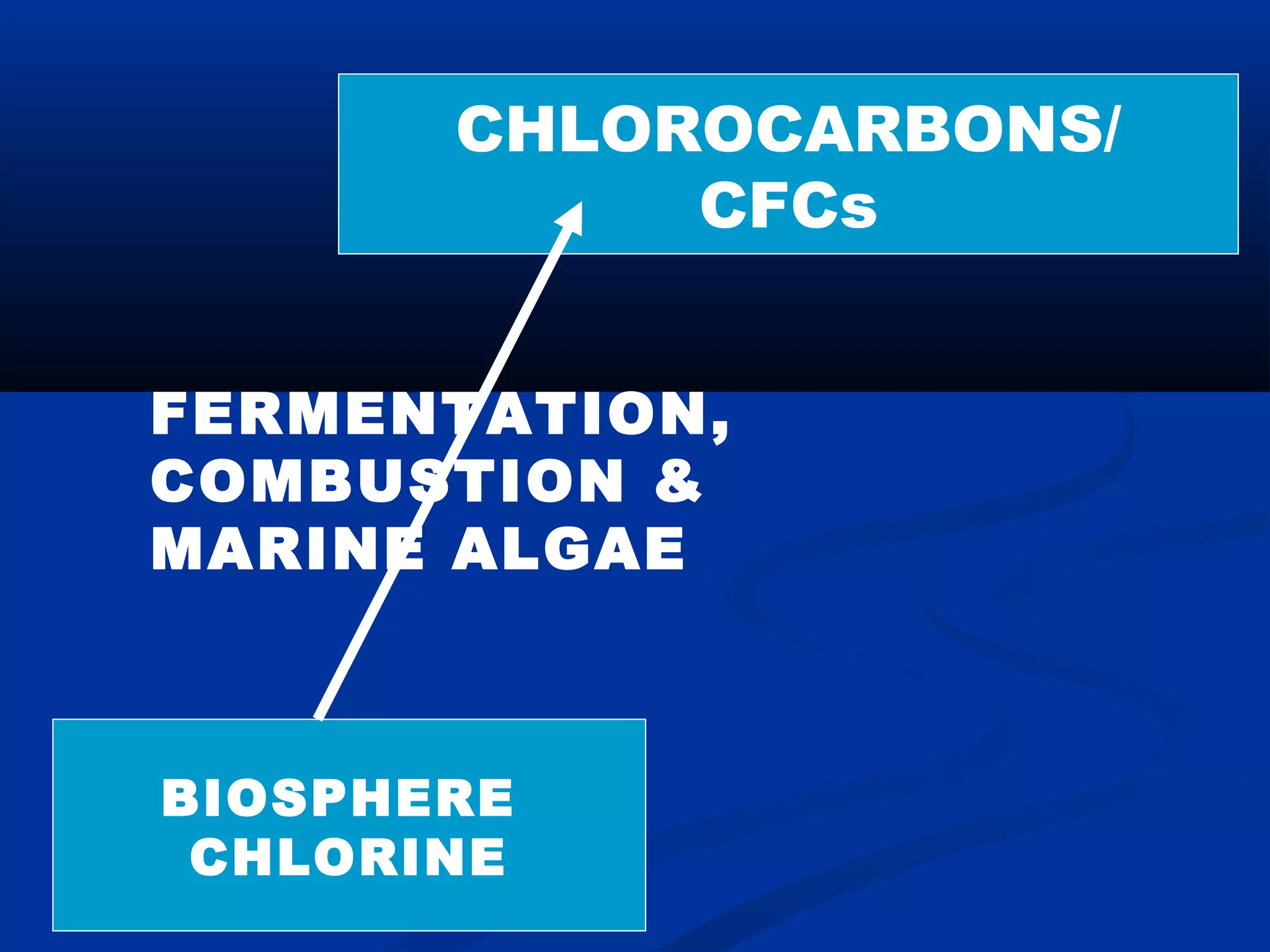
Chlorine, an essential element found in soils, minerals, plants, and seawater, plays a crucial role in various applications, including as a disinfectant and in the manufacturing of numerous products. Discovered by Carl Wilhelm Scheele in 1774 and recognized as an element in 1807, chlorine is highly reactive and commonly occurs as compounds such as sodium chloride. Though not a nutrient, chlorine significantly influences human health and the environment, maintaining the balance of bodily fluids and aiding in digestion.