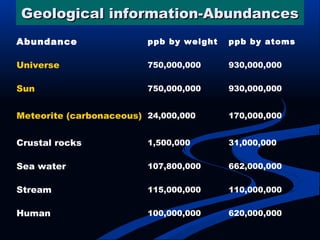
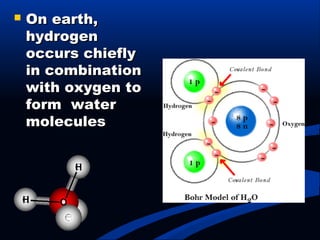
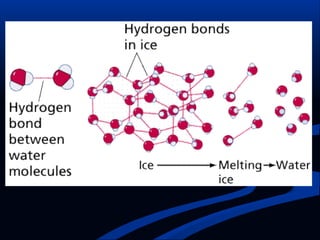
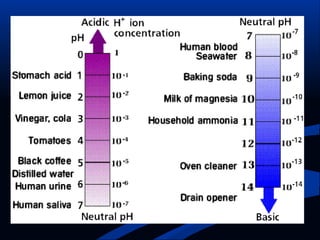
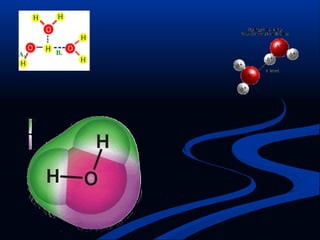
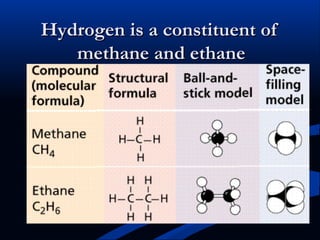
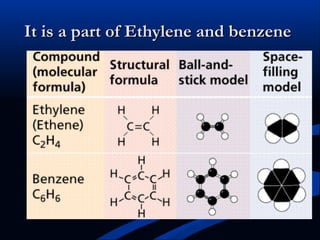
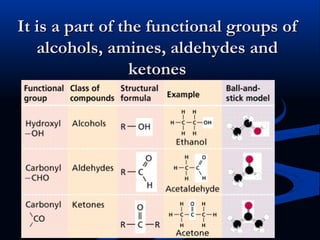
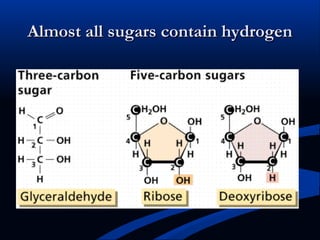
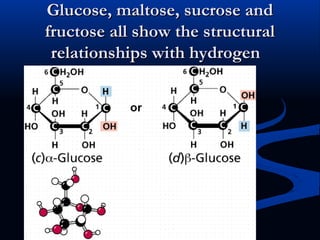
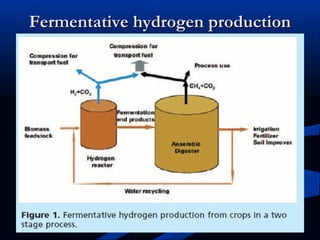
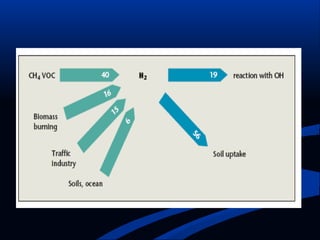
Hydrogen is a colorless, tasteless, and odorless gas, known as the first element of the periodic table and is the simplest and lightest atom. It plays a vital role in various biogeochemical cycles, exists primarily as a diatomic molecule (H2), and is essential for the formation of water and organic compounds. The document also discusses isotopes of hydrogen, its role in the universe, its occurrence in nature, and its importance in chemistry and biology.