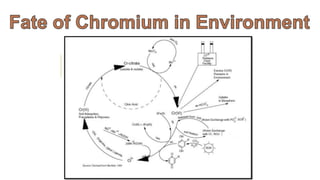
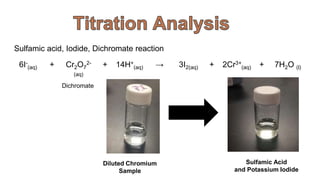
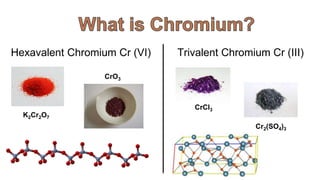
1. The document discusses hexavalent chromium [Cr(VI)] and trivalent chromium [Cr(III)], describing levels of Cr(VI) detected in California groundwater and regulations to limit it.
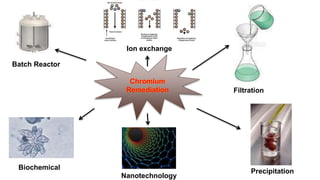
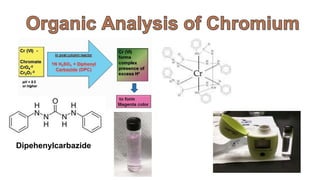
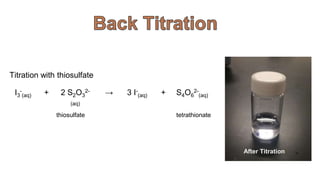
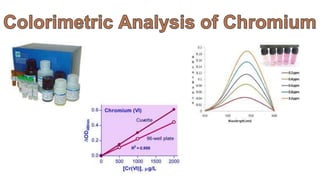
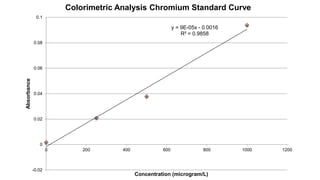
2. Methods for treating chromium-contaminated water are reviewed, including precipitation, filtration, ion exchange, and nanotechnology. Analytical techniques like colorimetric analysis and back titration are also summarized.
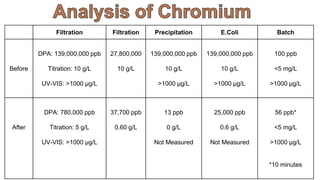
3. Results are presented from treating a high-level chromium sample using various treatment methods, showing reductions in chromium concentration but not reaching regulated levels.


![1. Cr (VI) detected in 3342/5477
wells tested
2. Los Angeles [Cr6+] > 10 μg/L
3. 2104 CA to limit Cr6+ to 10 ppb
4. Hinkley, CA had Cr6+ levels
of 31.8 ppb](https://image.slidesharecdn.com/5488d5fc-cc00-435a-980b-18c18e7464cc-170113001837/85/Chromium-ppt-4-320.jpg)