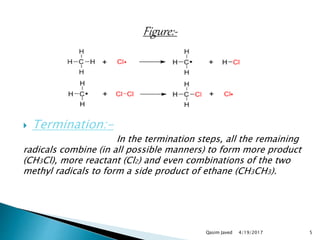
The document discusses chlorination, defining it as a halogenation reaction involving chlorine, particularly in water treatment. It outlines the three steps of chlorination—initiation, propagation, and termination—along with the reactions with methane, benzene, and alkenes, and notes that catalysts like aluminum chloride and iron chloride are used. Additionally, it highlights that chlorination produces chlorobenzene and is exothermic, emphasizing the importance of chlorine as a disinfectant in water.