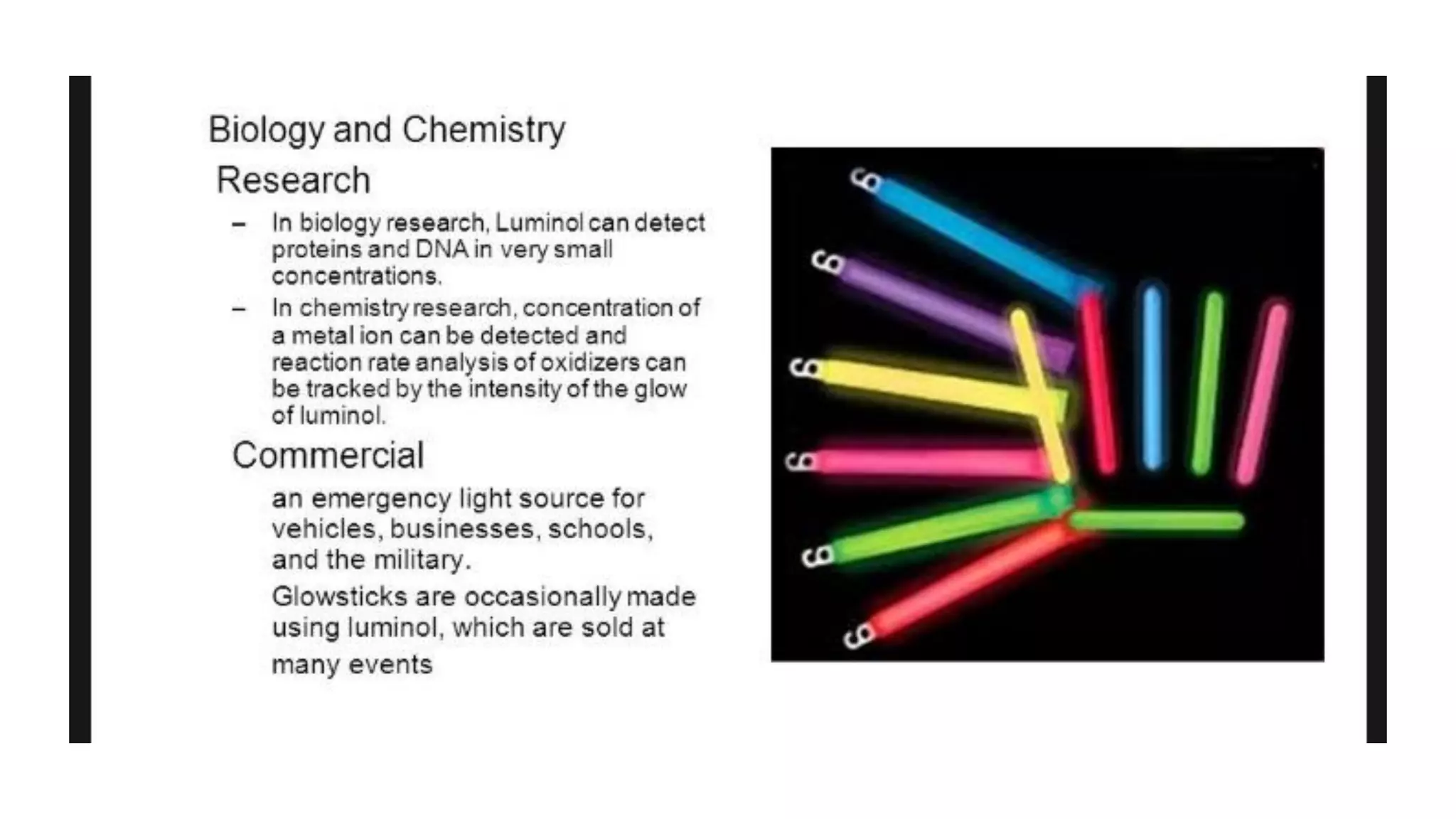
Chemiluminescence is the production of light from a chemical reaction. It occurs when two chemicals react to form an excited intermediate that releases energy in the form of photons as it decays to the ground state. Examples include fireflies, where the oxidation of luciferin produces light, and luminol, which was the first chemiluminescent molecule discovered. Applications include detecting gases, metal ions, and blood at crime scenes using luminol.