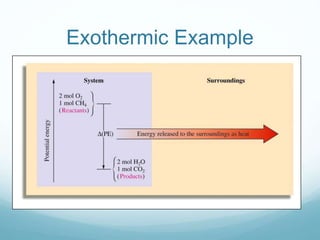
The document discusses thermochemistry and thermodynamics concepts. It defines key terms like:
- Thermodynamics is the study of energy and its interconversions.
- Energy can exist in potential or kinetic forms. Potential energy is due to position or composition, while kinetic energy depends on an object's mass and velocity.
- Enthalpy (H) is the combination of a system's internal energy and the work done on or by the system. It is a state function like internal energy.
- Calorimetry involves measuring heat changes using devices like coffee cup or bomb calorimeters. It relates heat to changes in temperature and heat capacity.