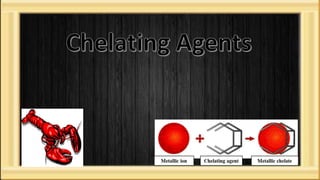
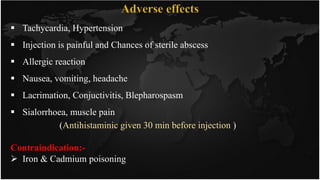
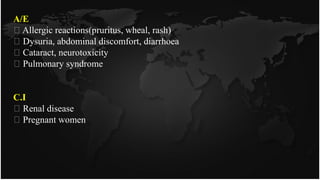
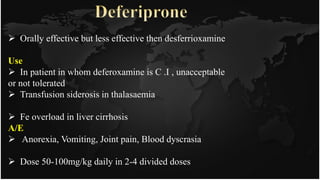
This document discusses heavy metal poisoning and chelating agents used to treat it. It provides details on common heavy metals that cause poisoning like lead, mercury, and arsenic. It then explains the process of chelation where chelating agents form stable, water soluble complexes with metal ions to facilitate their excretion. Several chelating agents are mentioned, including BAL, EDTA, penicillamine, deferiprone, and desferioxamine, along with their mechanisms of action, uses, and side effects in treating heavy metal poisoning.