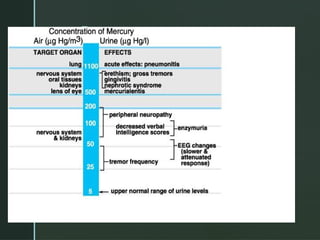
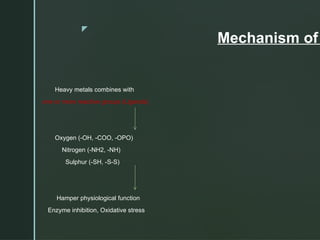
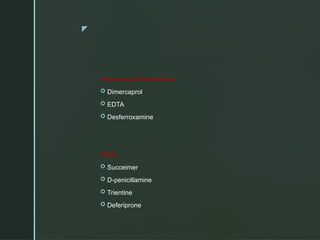
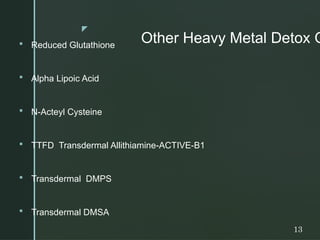
The document discusses heavy metals, their sources, and the harmful effects they have on human health, including organ damage, oxidative stress, and carcinogenic properties. It details various chelating agents used to detoxify the body from heavy metals, their mechanisms, routes of administration, and side effects. Notable chelates include dimercaprol, penicillamine, and EDTA, each with specific applications, advantages, and contraindications.