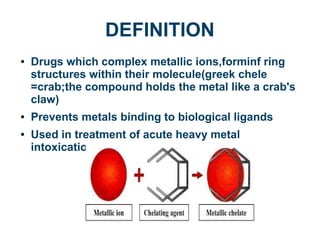
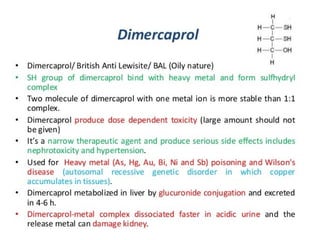
Chelating agents are drugs that form ring structures by binding to metallic ions. They are used to treat heavy metal intoxications by preventing metals from binding to biological ligands. An ideal chelator has the ability to reach sites of metal storage and form stable, non-toxic complexes that are excreted easily. It should have low affinity for essential metals like calcium and zinc. Common chelating agents include penicillamine, trientine, EDTA, deferoxamine, deferiprone, succimer, DMPS, and dimercaprol. Each agent has different mechanisms of action, routes of administration, targeted metals, and side effect profiles. Chelation therapy helps mobilize and eliminate toxic heavy metals from