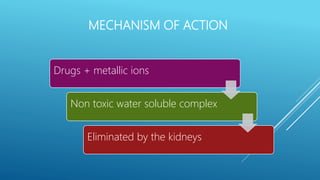
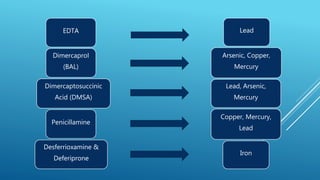
Chelating agents are drugs that form complexes with heavy metals to facilitate their removal from the body. They work by binding metals like lead, arsenic, and mercury to create non-toxic, water-soluble complexes that can be excreted in urine. Common chelating agents include dimercaprol, dimercaptosuccinic acid, dimercaptopropane sulfonic acid, disodium edetate, penicillamine, and desferrioxamine. Each agent has affinity for specific metals and is used to treat poisoning from those metals. The ideal chelating agent rapidly forms complexes that are non-toxic and easily excreted to safely eliminate metals from the body.