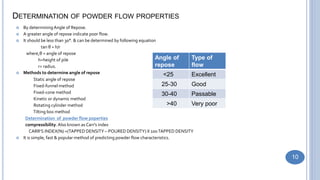
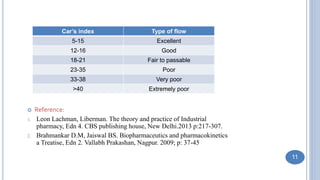
Preformulation studies characterize the physical and chemical properties of a drug to develop safe, effective, and stable dosage forms. Objectives include determining physico-chemical parameters, kinetics, stability, and compatibility with excipients. Bulk characterization studies crystallinity, polymorphism, and amorphous versus crystalline forms which impact properties like solubility and dissolution. Analytical methods like microscopy, thermal analysis, and spectroscopy are used to characterize solid forms. Other studies examine hygroscopicity, particle size, powder flow properties, and compressibility which influence processing and storage stability.