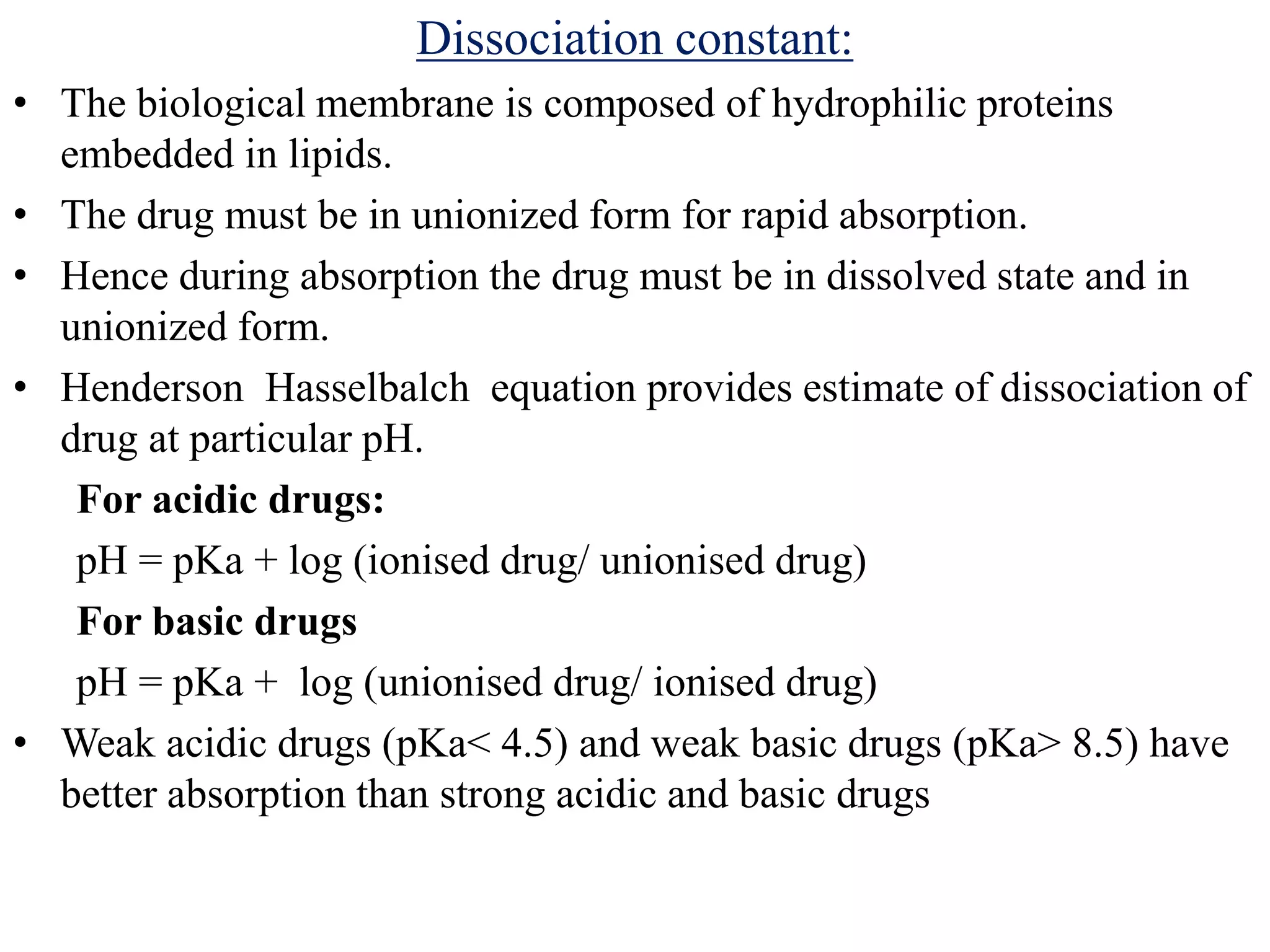
The document discusses preformulation studies, which characterize a new drug substance's physical and chemical properties to develop stable, safe dosage forms. Key goals are to establish the drug's properties, compatibility with excipients, and kinetic profile. Studies examine various physical parameters like appearance, particle size, flow properties, and chemical properties like solubility, polymorphism, hygroscopicity, and stability. This information guides dosage form development to deliver drugs efficiently and consistently.