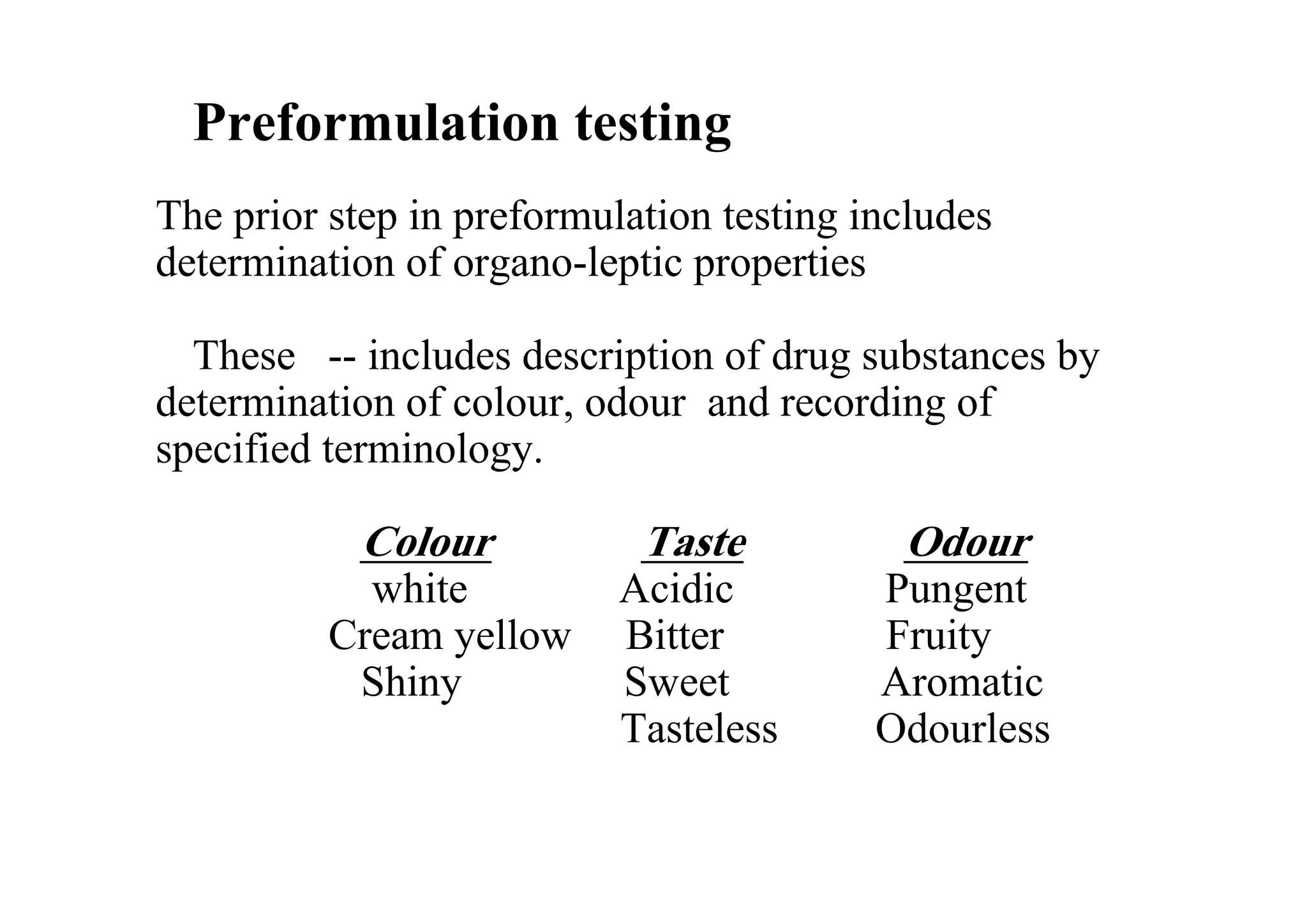
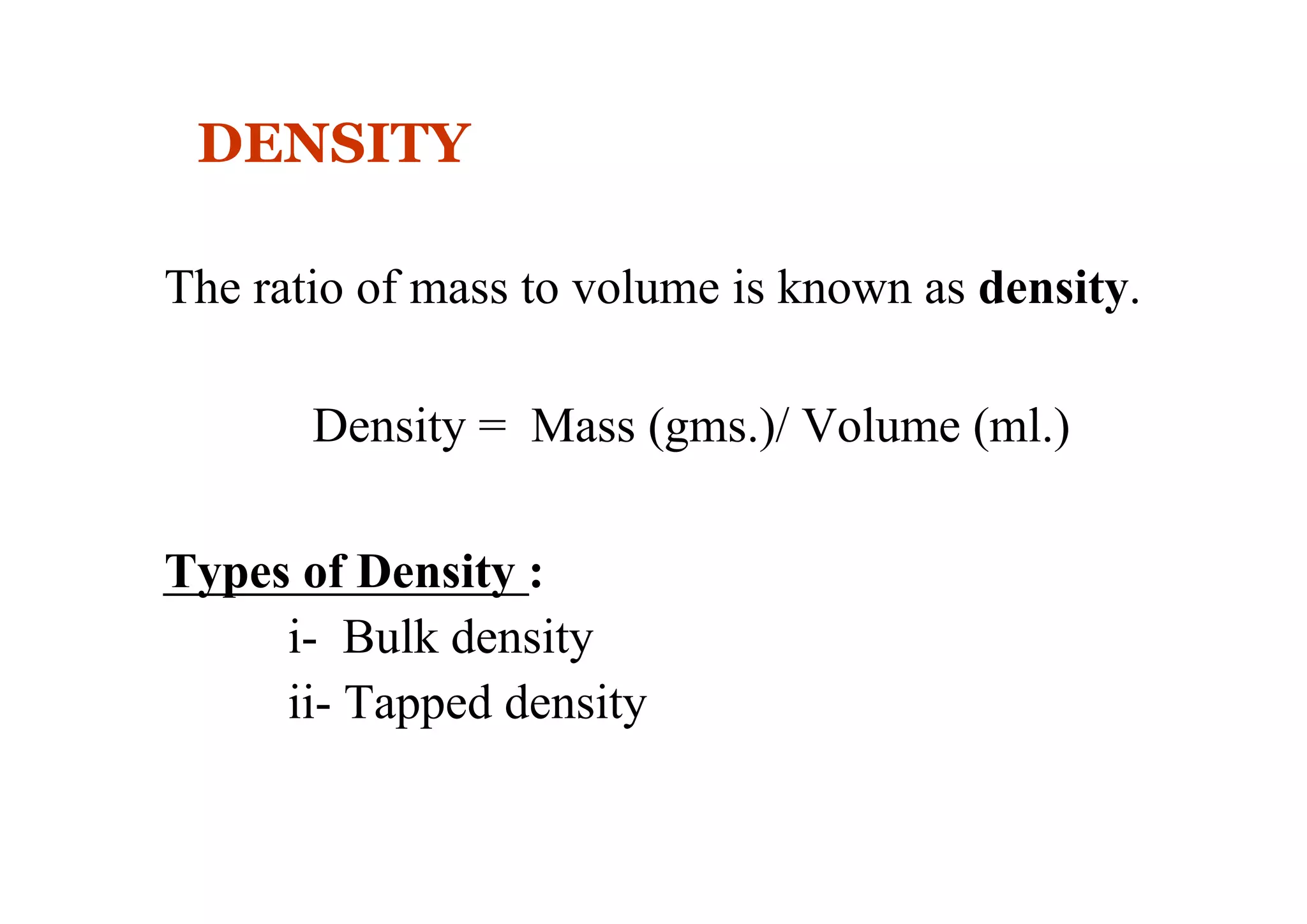
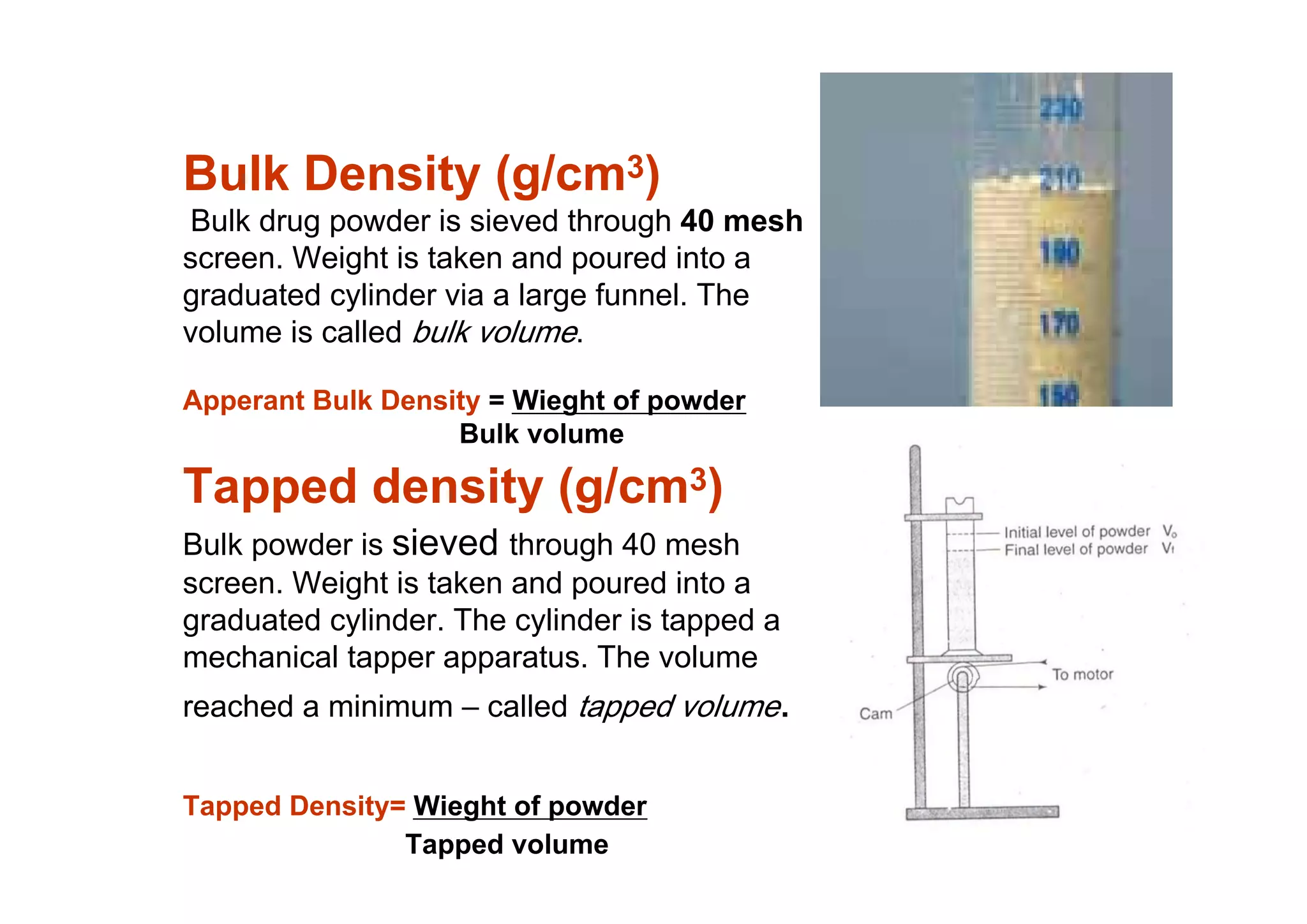
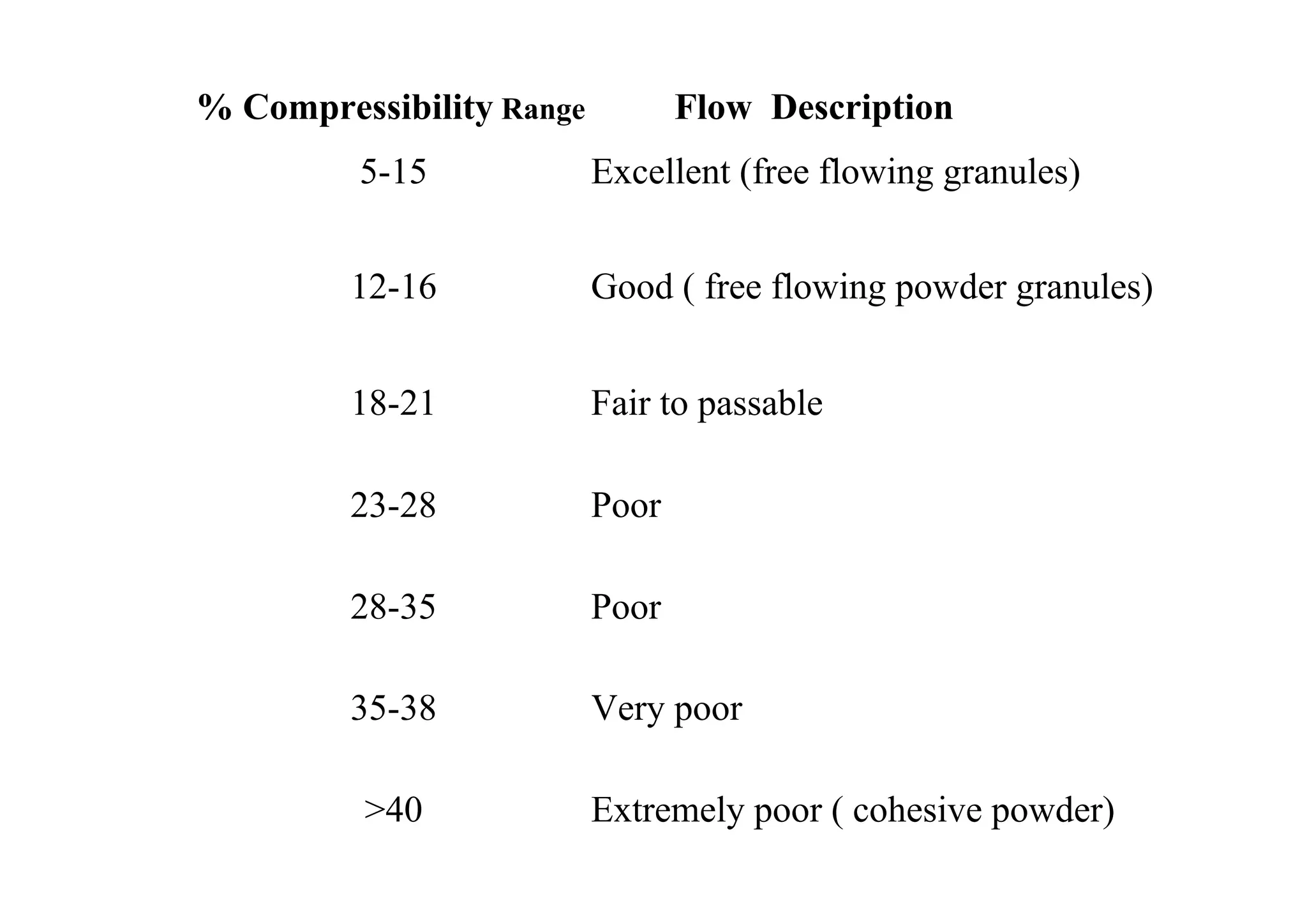
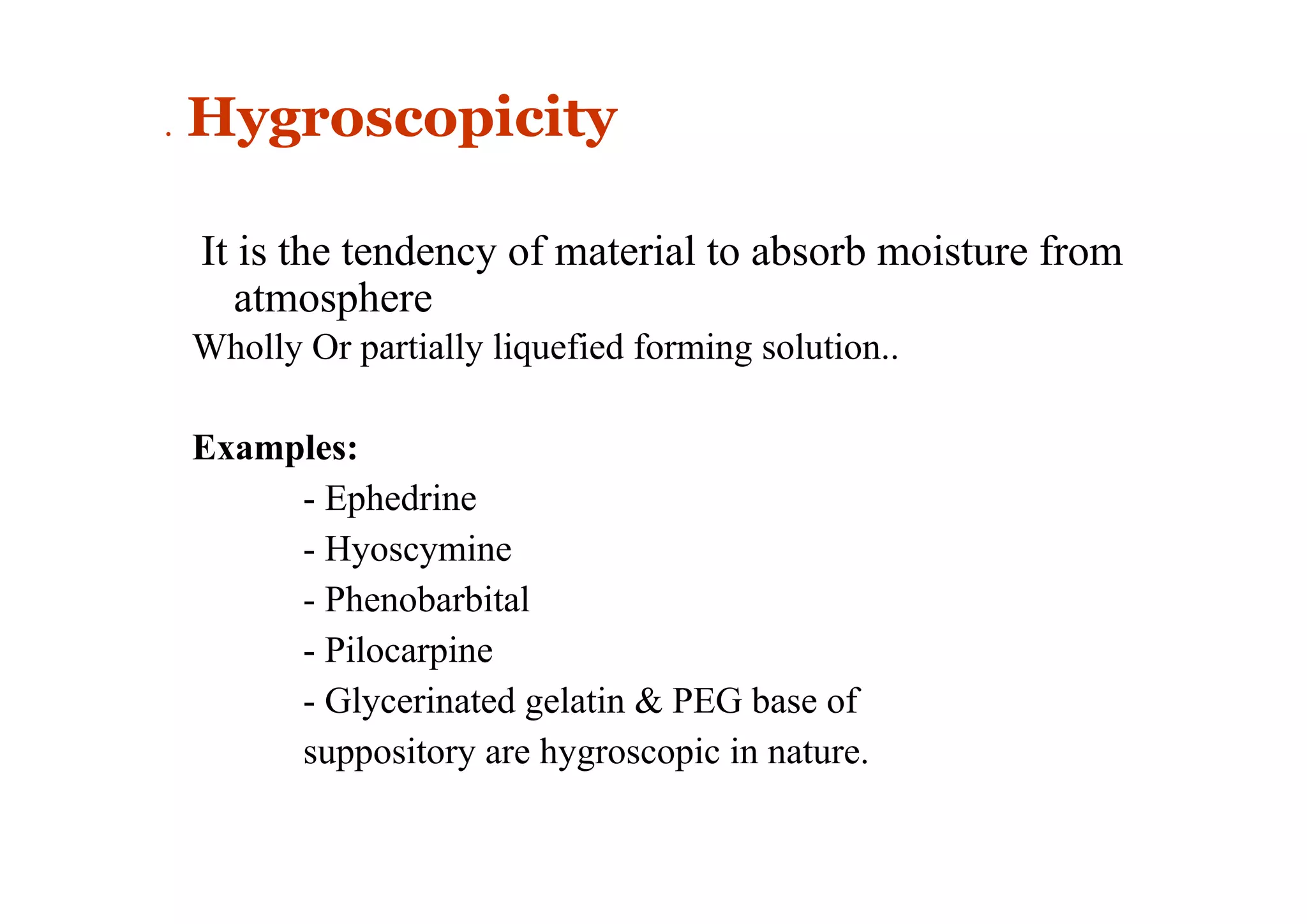
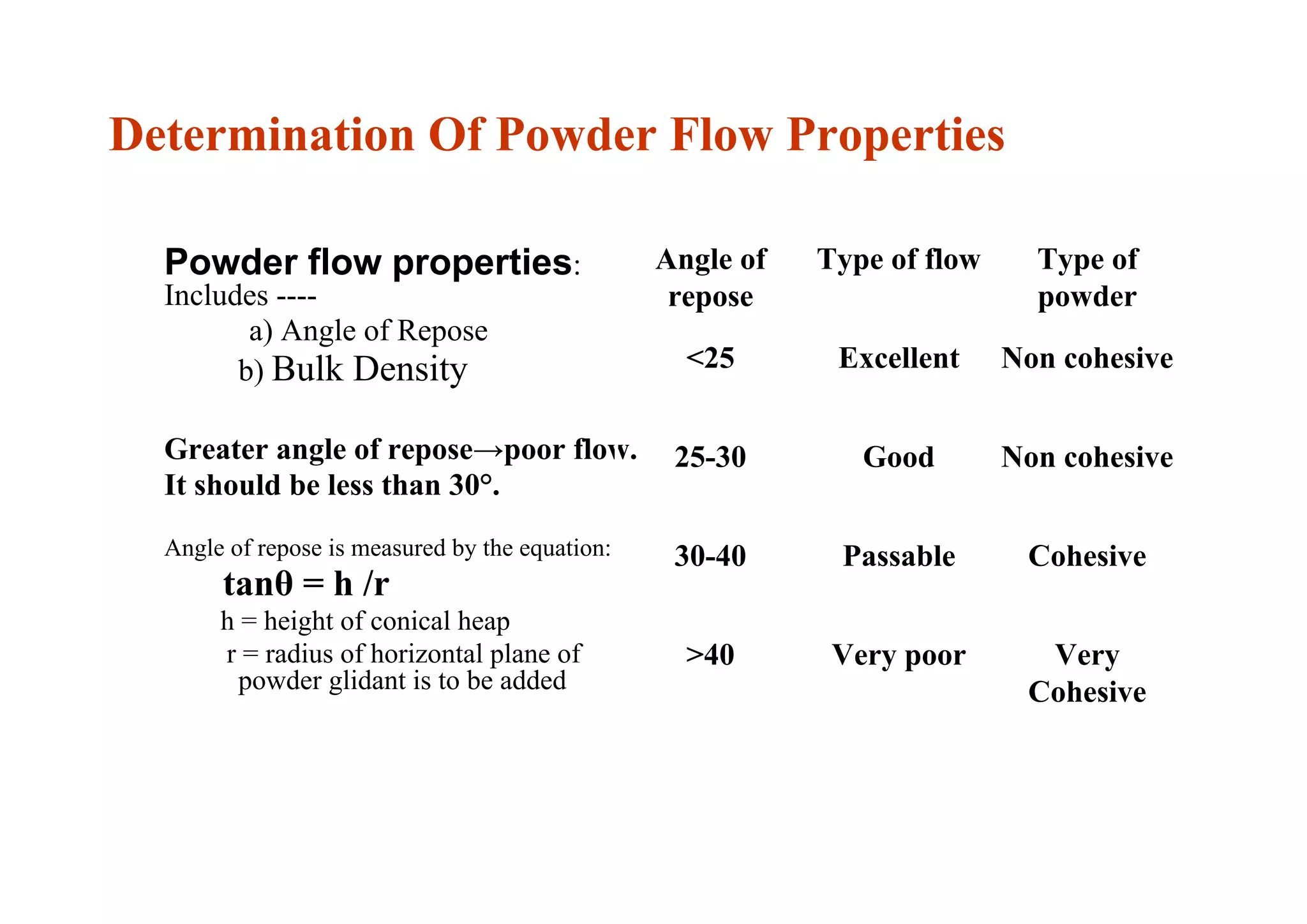
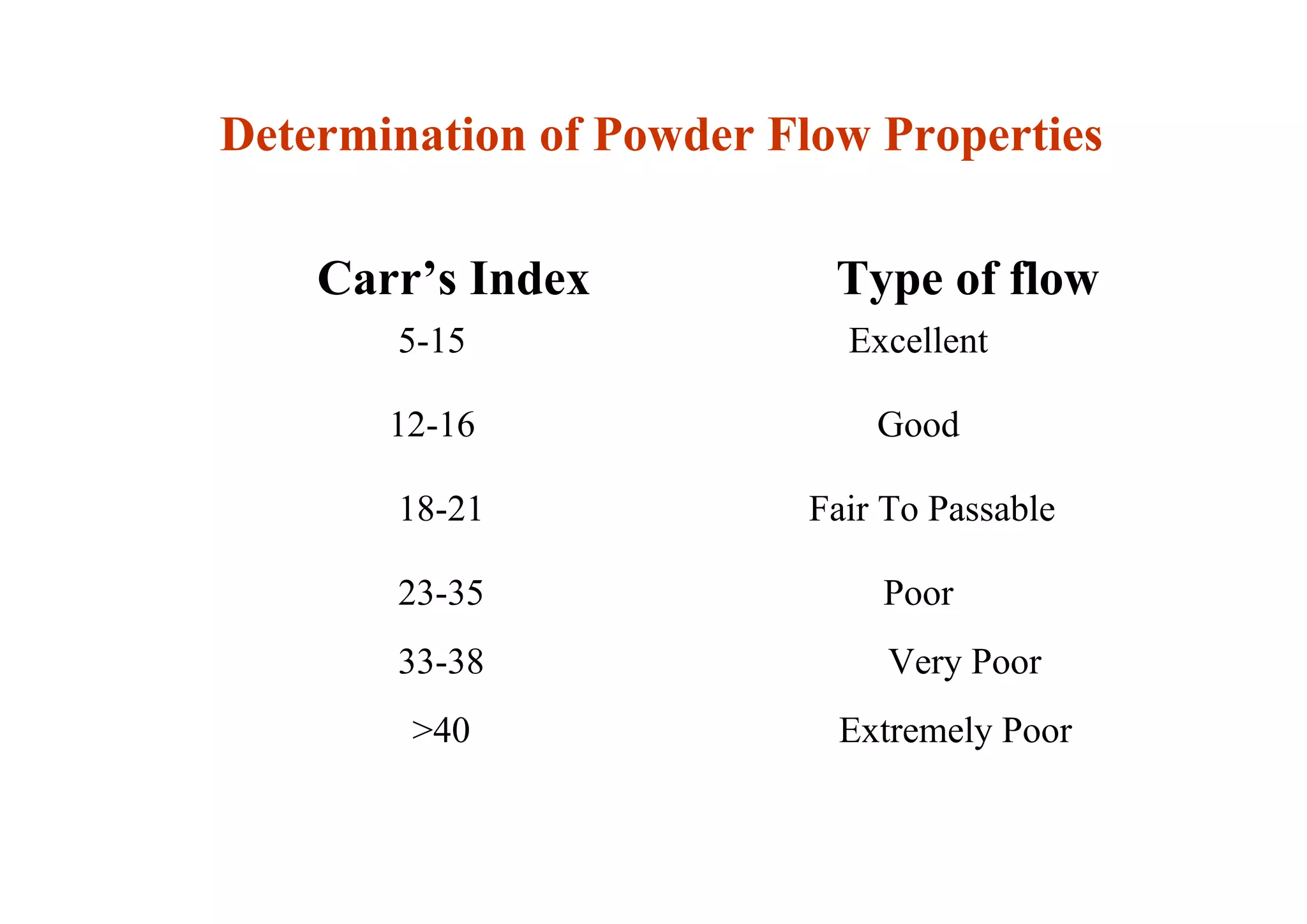
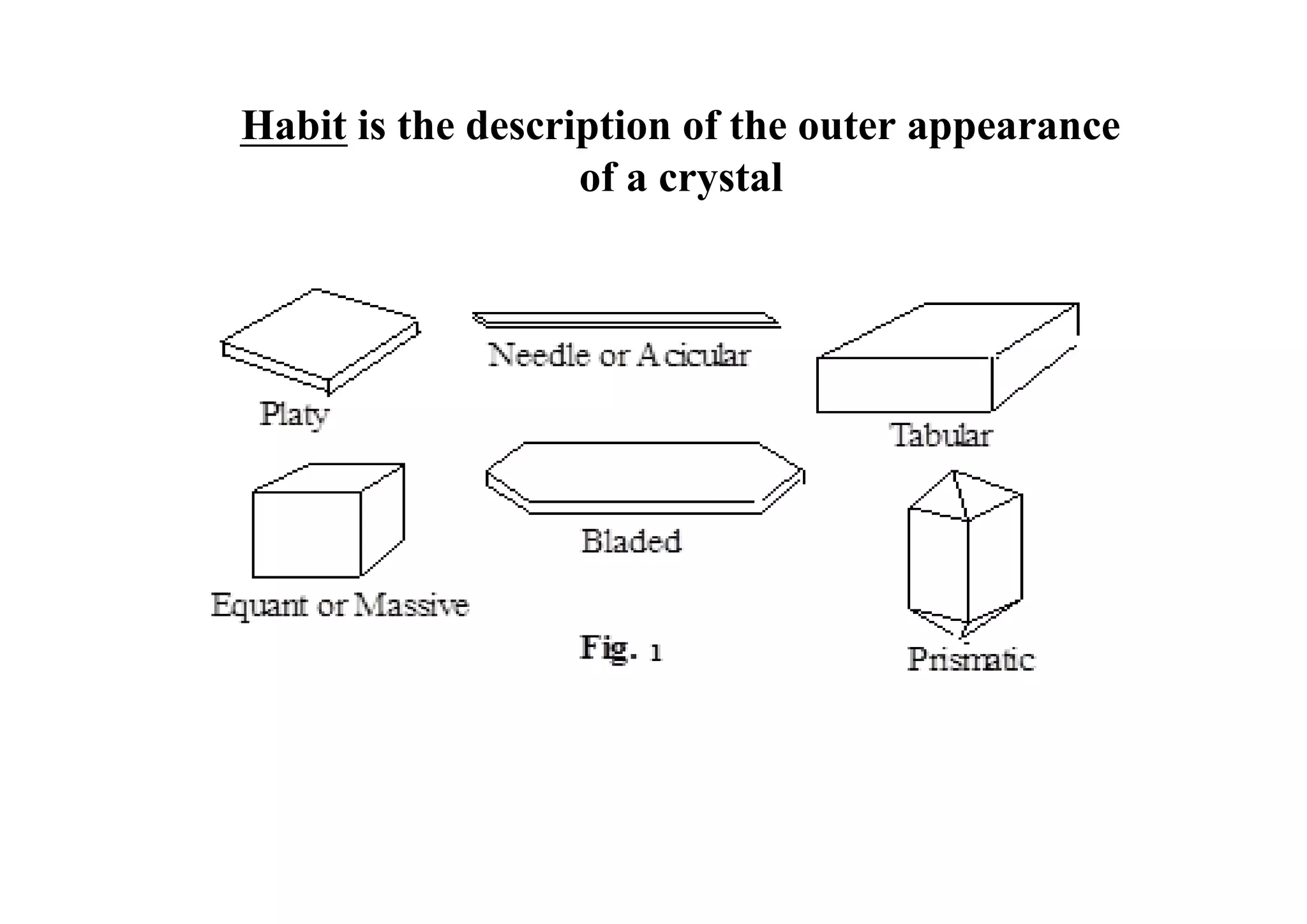
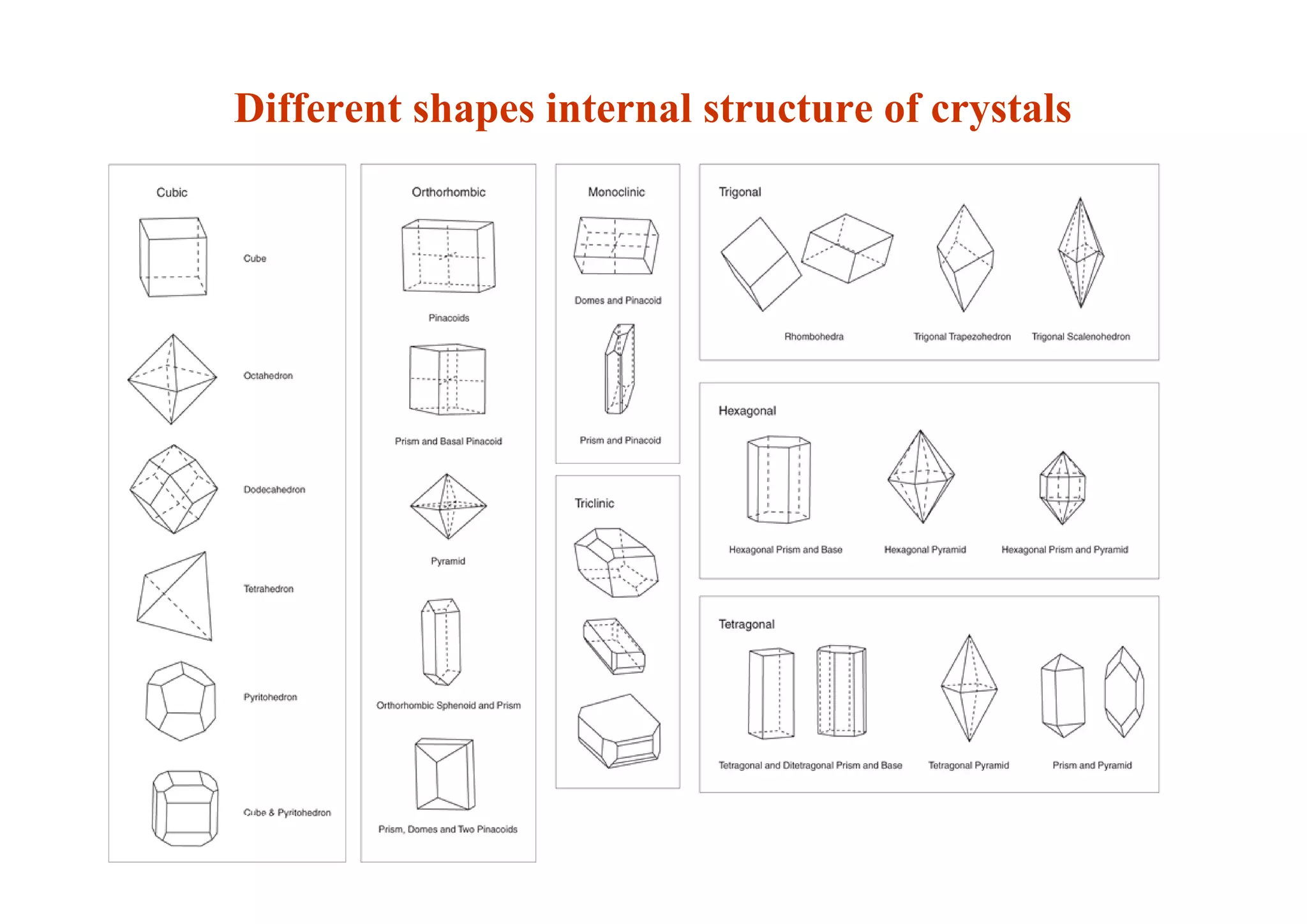
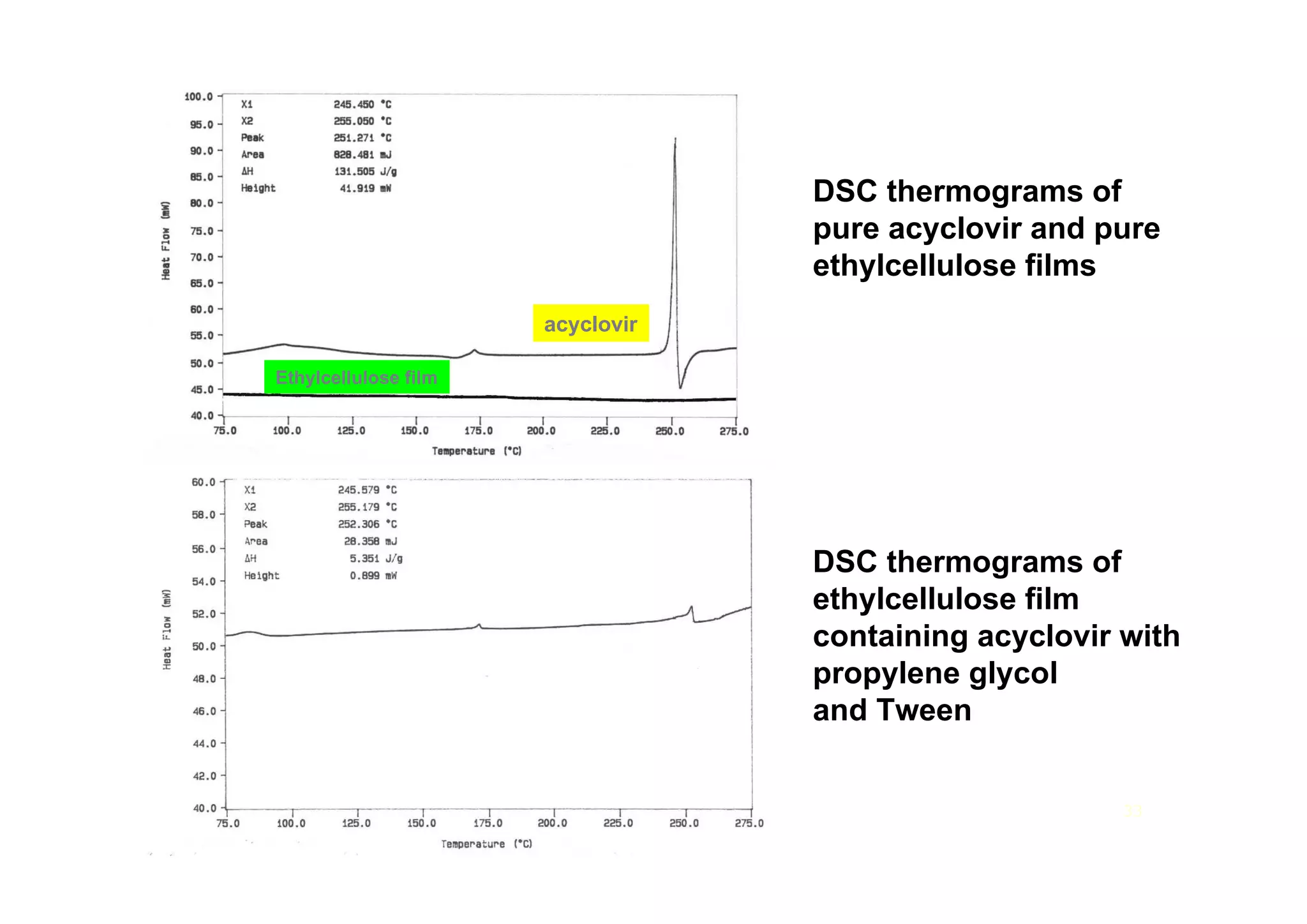
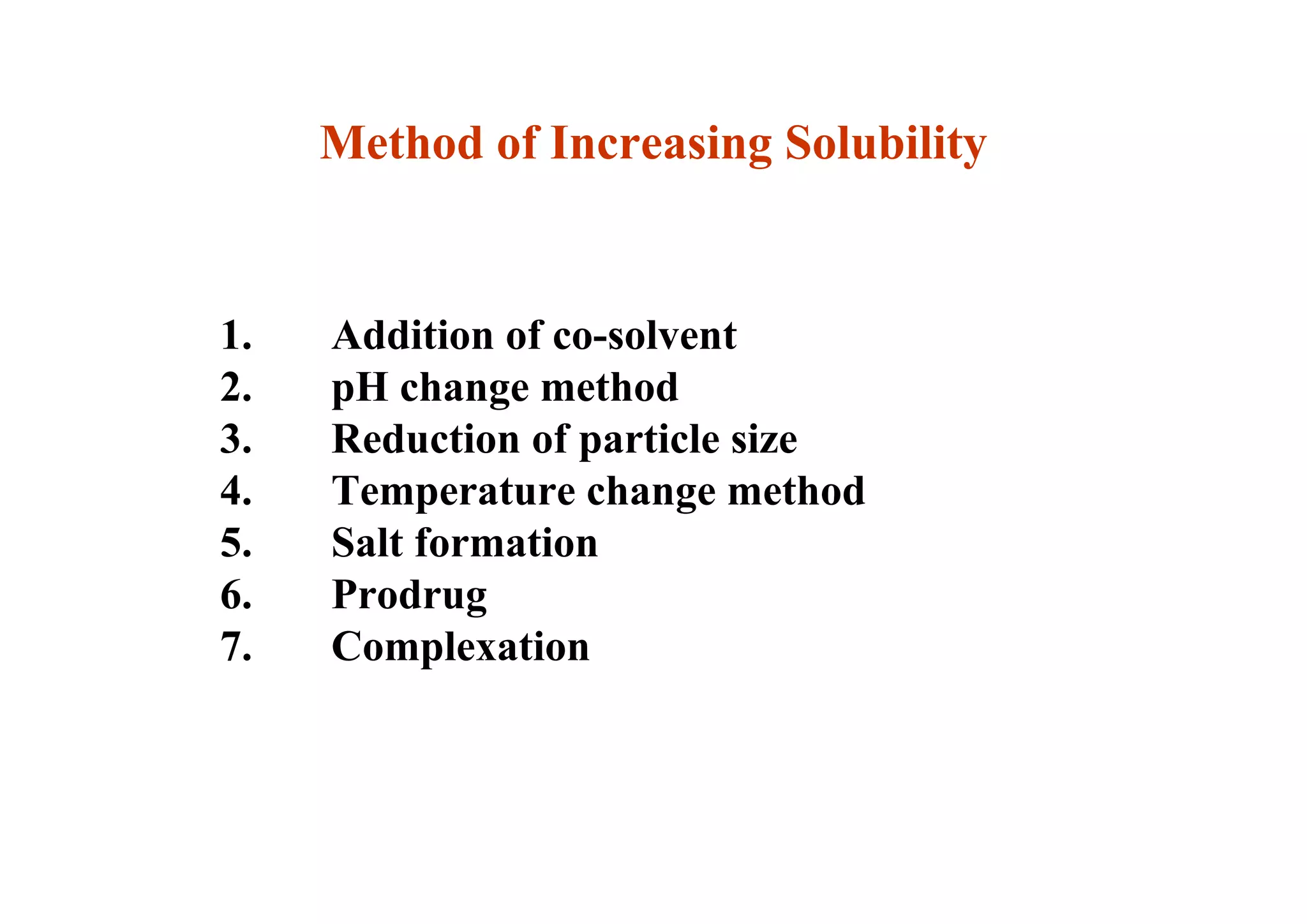
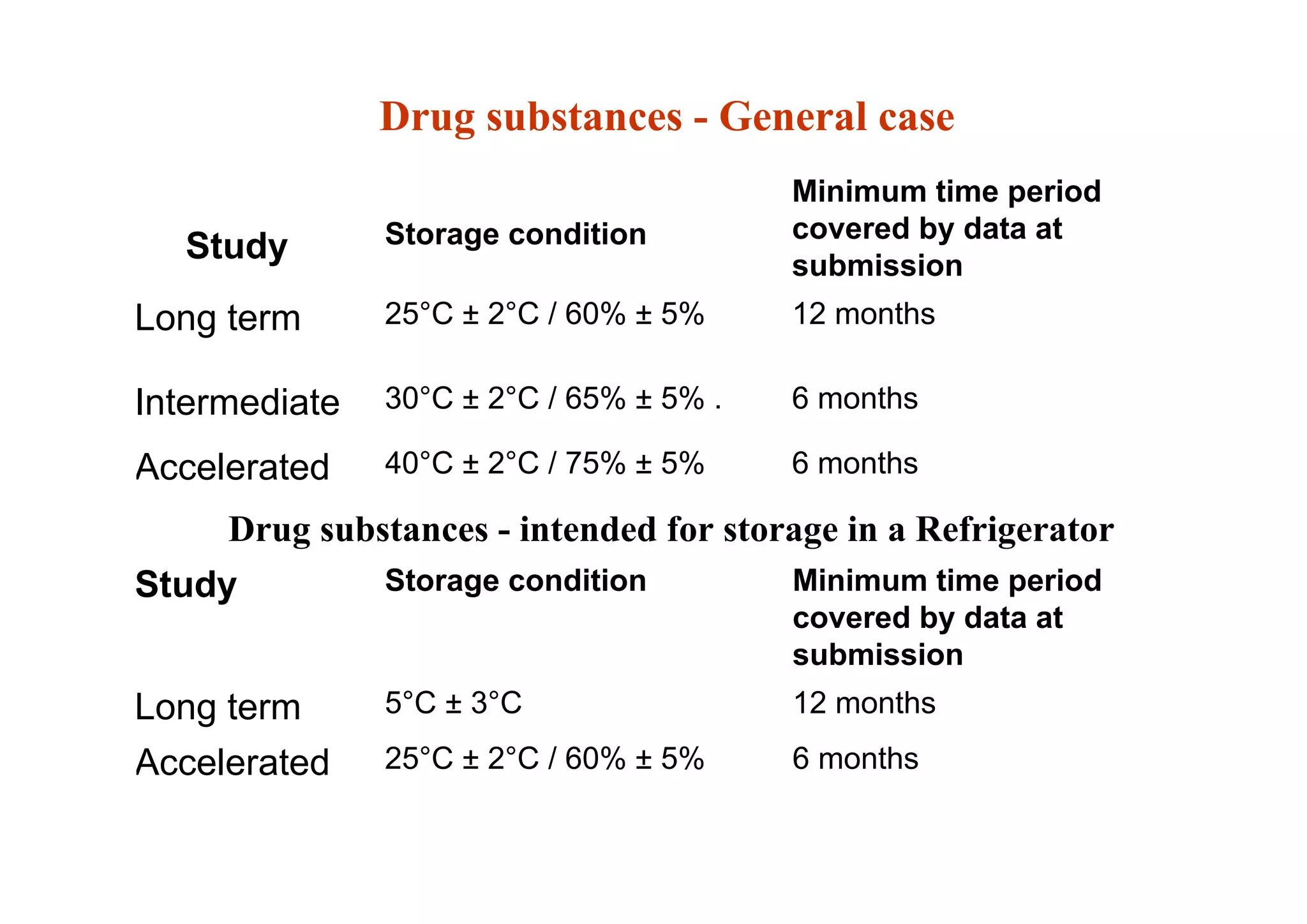
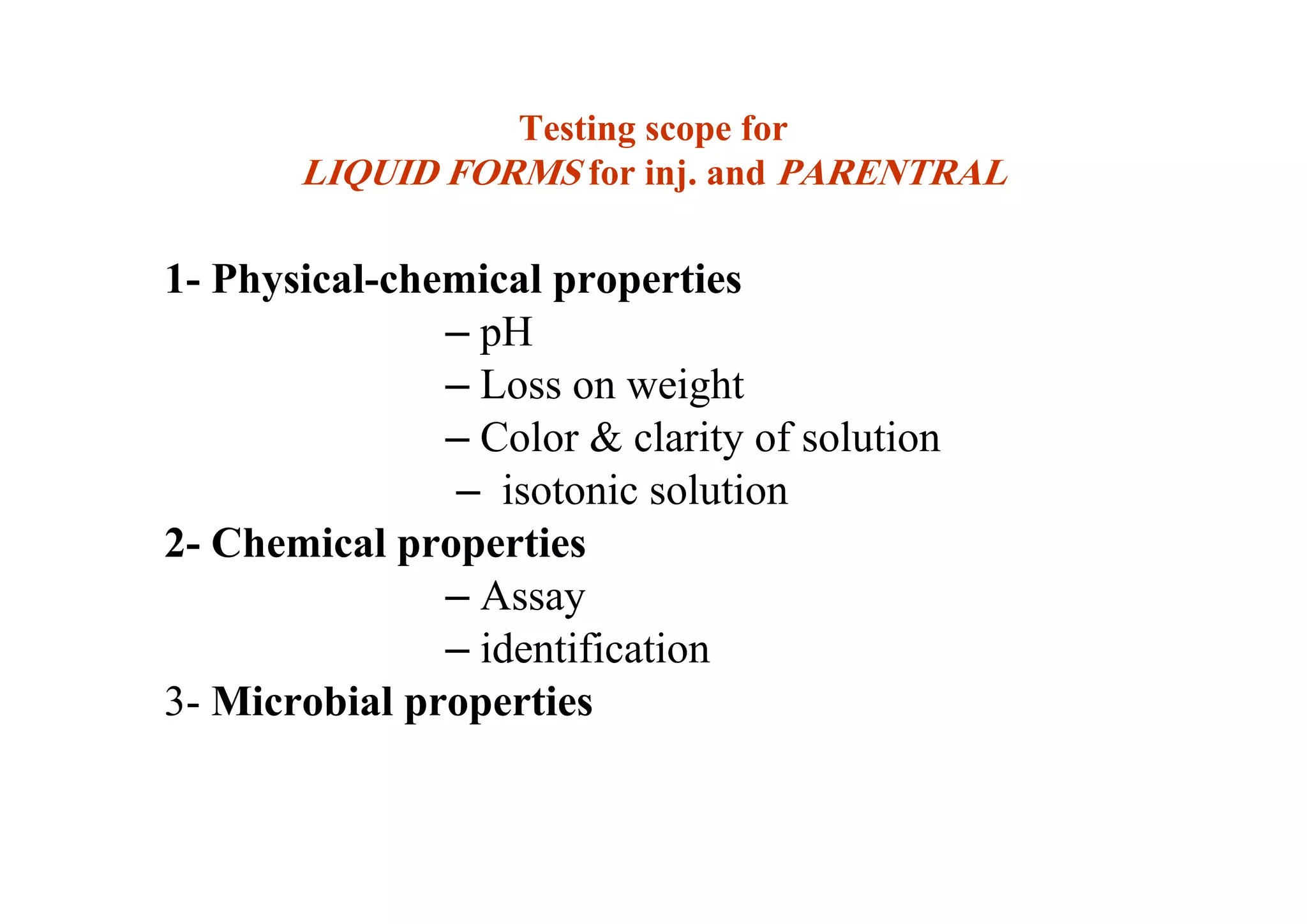
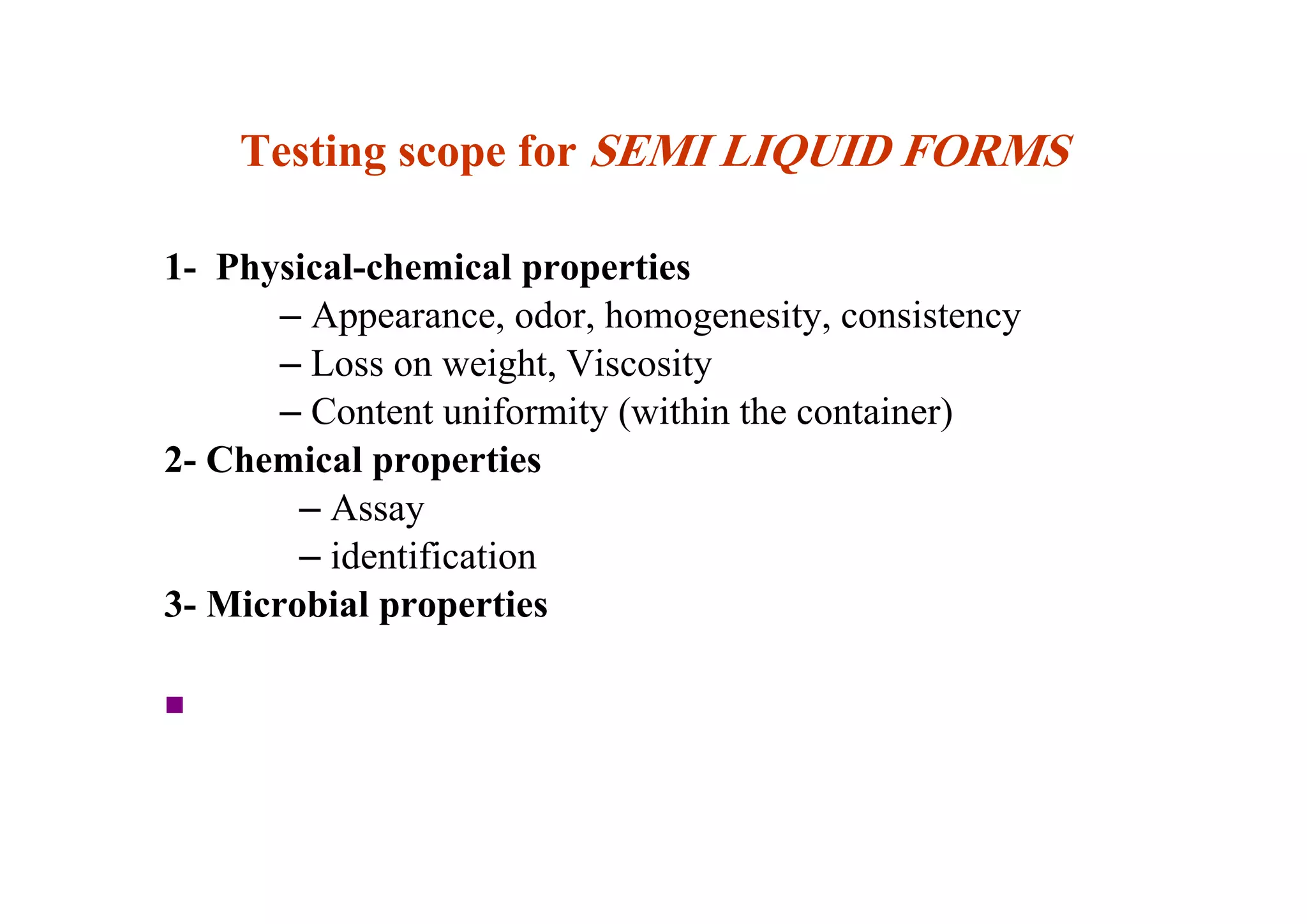
This document discusses pre-formulation, which involves investigating the physical and chemical properties of a drug substance alone or combined with excipients. The goal is to develop a stable, effective dosage form. Key tests described include identifying purity, density, compressibility, hygroscopicity, particle size, solid state properties, and compatibility with excipients. Properties like solubility, partition coefficient, and stability are also important to determine for pre-formulation.