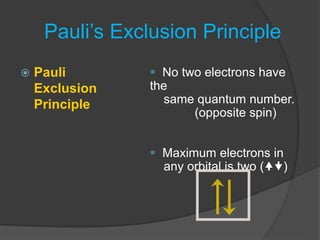
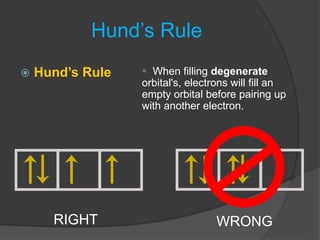
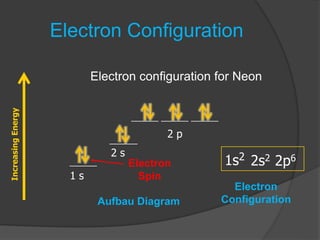
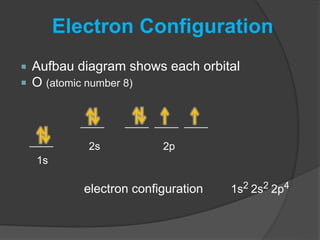
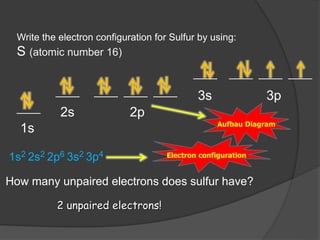
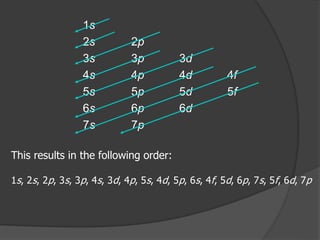
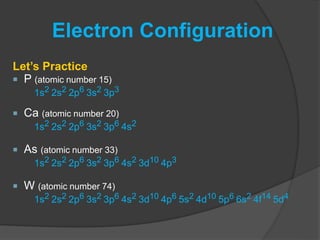
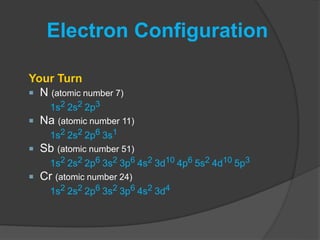
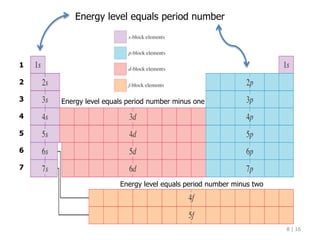
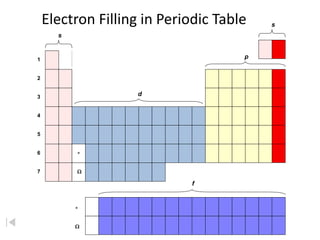
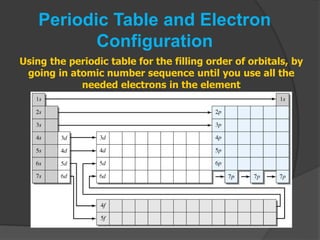
The document discusses the Pauli exclusion principle and electron configurations. It explains that according to the Pauli exclusion principle, no two electrons can have the same four quantum numbers. This means that within one orbital, electrons must have opposite spin, and one orbital can hold a maximum of two electrons. The document then discusses how electrons fill atomic orbitals according to the aufbau principle, from lowest to highest energy in a specific order, and provides examples of writing electron configurations.