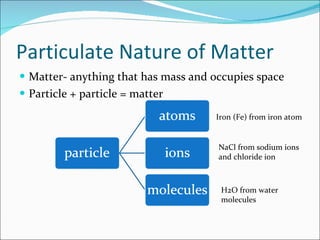
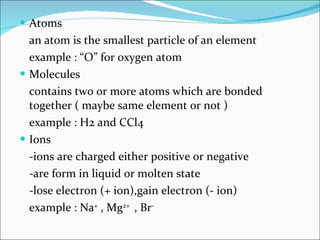
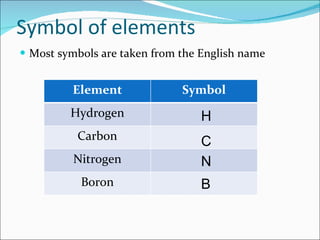
- Matter is composed of particles called atoms and molecules. Atoms are the smallest particles of elements, while molecules contain two or more bonded atoms.
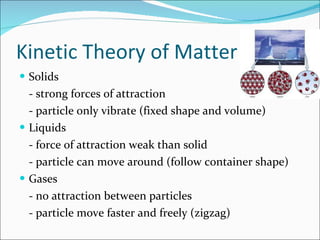
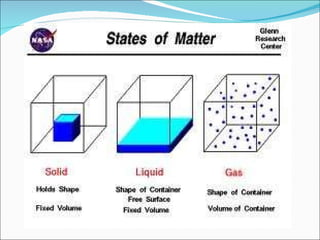
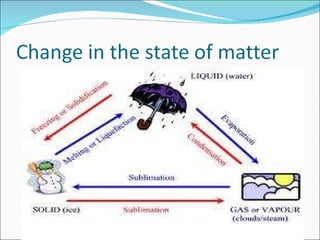
- The kinetic theory of matter describes the states of matter based on particle motion. Solids have fixed shapes, liquids have mobile particles that follow container shapes, and gases have freely moving particles.
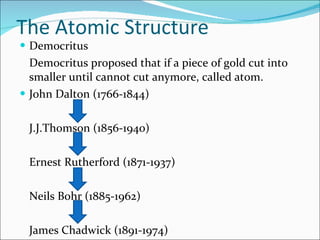
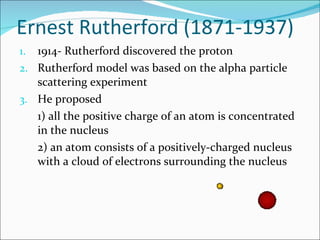
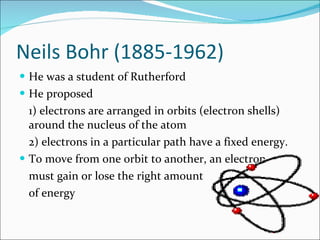
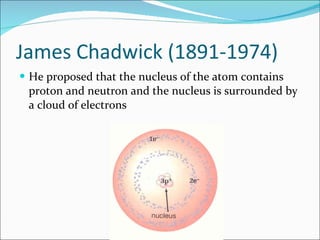
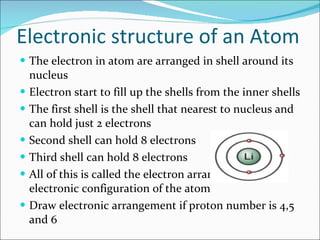
- The atomic structure has been discovered through the work of scientists like Dalton, Thomson, Rutherford, Bohr, and Chadwick. Modern atomic theory includes electrons orbiting a nucleus of protons and neutrons.
- Isotopes are atoms of the same element with different numbers of neutrons. Isotopes have identical chemical properties but different physical properties and masses