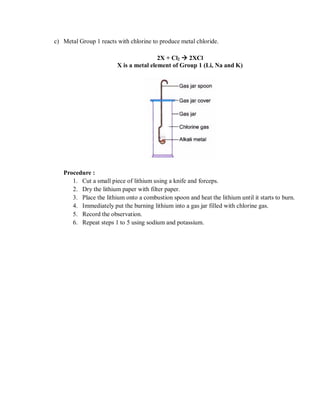
The document discusses the properties of two groups of elements - Group 18 noble gases and Group 1 alkali metals.
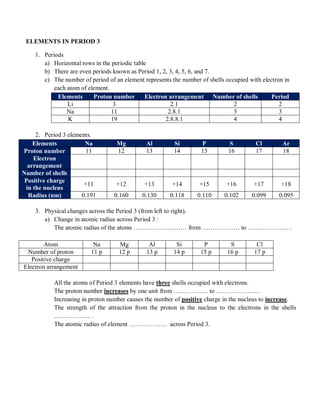
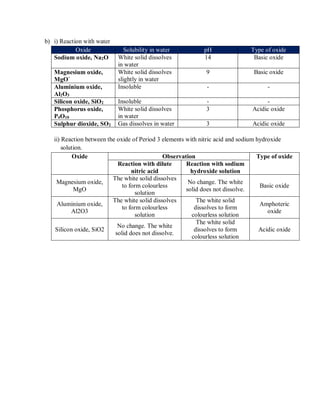
Group 18 consists of helium, neon, argon, krypton, xenon, and oganesson. Noble gases have full outer electron shells, making them chemically inert. Their melting and boiling points increase down the group as atomic size increases and van der Waals forces strengthen.
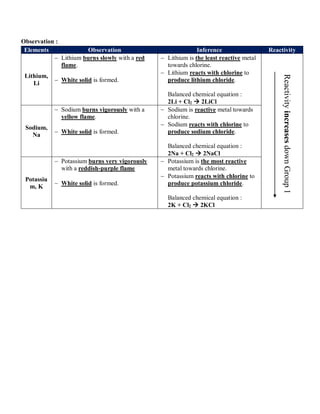
Group 1 includes lithium, sodium, potassium, cesium, and francium. Alkali metals react by losing one electron to form stable ions. Reactivity increases down the group as the valence electron is more loosely held. They react with water to form hydroxides and oxygen to form ox